Label: DRONABINOL capsule
-
NDC Code(s):
60687-375-01,
60687-375-11,
60687-375-21,
60687-386-11, view more60687-386-21, 60687-386-94
- Packager: American Health Packaging
- This is a repackaged label.
- Source NDC Code(s): 42858-867, 42858-868
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated March 27, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DRONABINOL CAPSULES, USP safely and effectively. See full prescribing information for DRONABINOL CAPSULES, USP.
DRONABINOL CAPSULES, USP for oral use, CIII
Initial U.S. Approval: 1985INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Anorexia Associated with Weight Loss in Adult Patients with AIDS(2.1):
- The recommended adult starting dosage is 2.5 mg orally twice daily, one hour before lunch and dinner.
- See the full prescribing information for dosage titration to manage adverse reactions and to achieve desired therapeutic effect.
Nausea and Vomiting Associated with Chemotherapy in Adult Patients Who Failed Conventional Antiemetics(2.2):
- The recommended starting dosage is 5 mg/m 2, administered 1 to 3 hours prior to the administration of chemotherapy, then every 2 to 4 hours after chemotherapy, for a total of 4 to 6 doses per day. Administer the first dose on an empty stomach at least 30 minutes prior to eating; subsequent doses can be taken without regard to meals.
- See the full prescribing information for dosage titration to manage adverse reactions and to achieve desired therapeutic effect.
DOSAGE FORMS AND STRENGTHS
- Capsules: 2.5 mg, 5 mg, 10 mg (3)
CONTRAINDICATIONS
- History of a hypersensitivity reaction to dronabinol or sesame oil (4)
WARNINGS AND PRECAUTIONS
- Neuropsychiatric Adverse Reactions: May cause psychiatric and cognitive effects and impair mental and/or physical abilities. Avoid use in patients with a psychiatric history. Monitor for symptoms and avoid concomitant use of drugs with similar effects. Inform patients not to operate motor vehicles or other dangerous machinery until they are reasonably certain that dronabinol capsules do not affect them adversely. (5.1)
- Hemodynamic Instability: Patients with cardiac disorders may experience hypotension, hypertension, syncope or tachycardia. Avoid concomitant use of drugs with similar effects and monitor for hemodynamic changes after initiating or increasing the dosage of dronabinol capsules. (5.2)
- Seizures and Seizure-like Activity: Weigh the potential risk versus benefits before prescribing dronabinol capsules to patients with a history of seizures, including those requiring anti-epileptic medication or with other factors that lower the seizure threshold. Monitor patients and discontinue if seizures occur. (5.3)
- Multiple Substance Abuse: Assess risk for abuse or misuse in patients with a history of substance abuse or dependence, prior to prescribing dronabinol capsules and monitor for the development of associated behaviors or conditions. (5.4)
- Paradoxical Nausea, Vomiting, or Abdominal Pain: Consider dose reduction or discontinuation, if worsening of symptoms while on treatment. (5.5)
ADVERSE REACTIONS
- Most common adverse reactions (≥3%) are: abdominal pain, dizziness, euphoria, nausea, paranoid reaction, somnolence, thinking abnormal, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Rhodes Pharmaceuticals at 1-888-827-0616, or FDA at 1-800-FDA-1088, or www.fda.gov/medwatch
DRUG INTERACTIONS
- Inhibitors and inducers of CYP2C9 and CYP3A4: May alter dronabinol systemic exposure; monitor for potential dronabinol-related adverse reactions or loss of efficacy. (7.3)
- Highly protein-bound drugs:Potential for displacement of other drugs from plasma proteins; monitor for adverse reactions to concomitant highly protein-bound drugs and narrow therapeutic index drugs (e.g., warfarin, cyclosporine, amphotericin B) when initiating or increasing the dosage of dronabinol capsules. (7.4)
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm. (8.1)
- Lactation: Advise HIV-infected women not to breastfeed due to the potential for HIV transmission. Weight should be monitored in breastfed infants of mothers with nausea and vomiting associated with cancer chemotherapy in whom breastfeeding is appropriate. (8.2)
- Geriatric Use:Elderly patients may be more sensitive to the neuropsychiatric and postural hypotensive effects. Consider a lower starting dose in elderly patients. (2.1, 2.2, 5.1, 5.2, 8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Anorexia Associated with Weight Loss in Adult Patients with AIDS
2.2 Nausea and Vomiting Associated with Cancer Chemotherapy in Adult Patients Who Failed Conventional Antiemetics
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Neuropsychiatric Adverse Reactions
5.2 Hemodynamic Instability
5.3 Seizures
5.4 Multiple Substance Abuse
5.5 Paradoxical Nausea, Vomiting, or Abdominal Pain
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Additive CNS Effects
7.2 Additive Cardiac Effects
7.3 Effect of Other Drugs on Dronabinol
7.4 Highly Protein-Bound Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Effect of CYP2C9 Polymorphism
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Appetite Stimulation
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
DRONABINOL CAPSULES, USP are indicated in adults for the treatment of:
- anorexia associated with weight loss in patients with Acquired Immune Deficiency Syndrome (AIDS).
- nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments.
-
2 DOSAGE AND ADMINISTRATION
2.1 Anorexia Associated with Weight Loss in Adult Patients with AIDS
Starting Dosage
The recommended adult starting dosage of dronabinol capsules is 2.5 mg orally twice daily, one hour before lunch and dinner.In elderly patients or patients unable to tolerate 2.5 mg twice daily, consider initiating dronabinol capsules at 2.5 mg once daily one hour before dinner or at bedtime to reduce the risk of central nervous system (CNS) symptoms [see Use in Specific Populations (8.5)].
Dosing later in the day may reduce the frequency of CNS adverse reactions. CNS adverse reactions are dose-related [see Warnings and Precautions (5.1)]; therefore, monitor patients and reduce the dosage as needed. If CNS adverse reactions of feeling high, dizziness, confusion, and somnolence occur, they usually resolve in 1 to 3 days and usually do not require dosage reduction. If CNS adverse reactions are severe or persistent, reduce the dosage to 2.5 mg in the evening or at bedtime.
Dosage Titration
If tolerated and further therapeutic effect is desired, the dosage may be increased gradually to 2.5 mg one hour before lunch and 5 mg one hour before dinner. Increase the dose of dronabinol capsules gradually in order to reduce the frequency of dose-related adverse reactions [see Warnings and Precautions (5.1)].Most patients respond to 2.5 mg twice daily, but the dose may be further increased to 5 mg one hour before lunch and 5 mg one hour before dinner, as tolerated to achieve a therapeutic effect.
Maximum Dosage: 10 mg twice daily.
2.2 Nausea and Vomiting Associated with Cancer Chemotherapy in Adult Patients Who Failed Conventional Antiemetics
Starting Dosage
The recommended starting dosage of dronabinol capsules is 5 mg/m 2, orally administered 1 to 3 hours prior to the administration of chemotherapy and then every 2 to 4 hours after chemotherapy, for a total of 4 to 6 doses per day.In elderly patients, consider initiating dronabinol capsules at 2.5 mg/m 2 once daily 1 to 3 hours prior to chemotherapy to reduce the risk of CNS symptoms [see Use in Specific Populations (8.5)].
Administer the first dose on an empty stomach at least 30 minutes before eating. Subsequent doses can be taken without regard to meals [see Clinical Pharmacology (12.3)].
The timing of dosing in relation to meal times should be kept consistent for each chemotherapy cycle, once the dosage has been determined from the titration process.
Dosage Titration
The dosage can be titrated to clinical response during a chemotherapy cycle or subsequent cycles, based upon initial response, as tolerated to achieve a clinical effect, in increments of 2.5 mg/m 2.The maximum dosage is 15 mg/m 2 per dose for 4 to 6 doses per day.
Adverse reactions are dose-related and psychiatric symptoms increase significantly at the maximum dosage [see Warnings and Precautions (5.1)].
Monitor patients for adverse reactions and consider decreasing the dose to 2.5 mg once daily 1 to 3 hours prior to chemotherapy to reduce the risk of CNS adverse reactions.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Dronabinol capsules are contraindicated in patients with a history of a hypersensitivity reaction to dronabinol or sesame oil. Reported hypersensitivity reactions to dronabinol capsules include lip swelling, hives, disseminated rash, oral lesions, skin burning, flushing, and throat tightness [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Neuropsychiatric Adverse Reactions
Psychiatric Adverse Reactions
Dronabinol has been reported to exacerbate mania, depression, or schizophrenia. Significant CNS symptoms followed oral doses of 0.4 mg/kg (28 mg per 70 kg patient) of dronabinol capsules in antiemetic studies.Prior to initiating treatment with dronabinol capsules, screen patients for a history of these illnesses. Avoid use in patients with a psychiatric history or, if the drug cannot be avoided, monitor patients for new or worsening psychiatric symptoms during treatment. Also, avoid concomitant use with other drugs that are associated with similar psychiatric effects.
Cognitive Adverse Reactions
Use of dronabinol capsules has been associated with cognitive impairment and altered mental state. Reduce the dose of dronabinol capsules or discontinue use of dronabinol capsules if signs or symptoms of cognitive impairment develop. Elderly patients may be more sensitive to the neurological and psychoactive effects of dronabinol capsules [see Use in Specific Populations (8.4, 8.5)].Hazardous Activities
Dronabinol capsules can cause and may impair the mental and/or physical abilities required for the performance of hazardous tasks such as driving a motor vehicle or operating machinery. Concomitant use of other drugs that cause dizziness, confusion, sedation, or somnolence such as CNS depressants may increase this effect (e.g., barbiturates, benzodiazepines, ethanol, lithium, opioids, buspirone, scopolamine, antihistamines, tricyclic antidepressants, other anticholinergic agents, muscle relaxants). Inform patients not to operate motor vehicles or other dangerous machinery until they are reasonably certain that dronabinol capsules do not affect them adversely.5.2 Hemodynamic Instability
Patients may experience occasional hypotension, possible hypertension, syncope, or tachycardia while taking dronabinol capsules [see Clinical Pharmacology (12.2)]. Patients with cardiac disorders may be at higher risk. Avoid concomitant use of other drugs that are also associated with similar cardiac effects (e.g., amphetamines, other sympathomimetic agents, atropine, amoxapine, scopolamine, antihistamines, other anticholinergic agents, amitriptyline, desipramine, other tricyclic antidepressants). Monitor patients for changes in blood pressure, heart rate, and syncope after initiating or increasing the dosage of dronabinol capsules.
5.3 Seizures
Seizure and seizure-like activity have been reported in patients receiving dronabinol.
Weigh this potential risk against the benefits before prescribing dronabinol capsules to patients with a history of seizures, including those receiving anti-epileptic medication or with other factors that can lower the seizure threshold. Monitor patients with a history of seizure disorders for worsened seizure control during dronabinol capsules therapy.
If a seizure occurs, advise patients to discontinue dronabinol capsules and contact a healthcare provider immediately.
5.4 Multiple Substance Abuse
Patients with a history of substance abuse or dependence, including marijuana or alcohol, may be more likely to abuse dronabinol capsules as well.
Assess each patient's risk for abuse or misuse prior to prescribing dronabinol capsules and monitor patients with a history of substance abuse during treatment with dronabinol capsules for the development of these behaviors or conditions.
5.5 Paradoxical Nausea, Vomiting, or Abdominal Pain
Nausea, vomiting, or abdominal pain can occur during treatment with synthetic delta-9‑tetrahydrocannabinol (delta-9-THC), the active ingredient in dronabinol capsules. In some cases, these adverse reactions were severe (e.g., dehydration, electrolyte abnormalities) and required dose reduction or drug discontinuation. Symptoms are similar to cannabinoid hyperemesis syndrome (CHS), which is described as cyclical events of abdominal pain, nausea, and vomiting in chronic, long-term users of delta-9-THC products.
Because patients may not recognize these symptoms as abnormal, it is important to specifically ask patients or their caregivers about the development of worsening of nausea, vomiting, or abdominal pain while being treated with dronabinol capsules. Consider dose reduction or discontinuing dronabinol capsules if a patient develops worsening nausea, vomiting, or abdominal pain while on treatment.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following serious adverse reactions are described below and elsewhere in the labeling.
- Neuropsychiatric Adverse Reactions [see Warnings and Precautions (5.1)]
- Hemodynamic Instability [see Warnings and Precautions (5.2)]
- Seizures [see Warnings and Precautions (5.3)]
- Paradoxical Nausea, Vomiting, and Abdominal Pain [see Warnings and Precautions (5.5)]
Studies of AIDS-related weight loss included 157 patients receiving dronabinol capsules at a dose of 2.5 mg twice daily and 67 receiving placebo. Studies of nausea and vomiting related to cancer chemotherapy included 317 patients receiving dronabinol capsules and 68 receiving placebo. In the tables below is a summary of the adverse reactions in 474 patients exposed to dronabinol capsules in studies.
Studies of different durations were combined by considering the first occurrence of events during the first 28 days.
A cannabinoid dose-related "high" (easy laughing, elation, and heightened awareness) has been reported by patients receiving dronabinol capsules in both the antiemetic (24%) and the lower dose appetite stimulant clinical trials (8%). The most frequently reported adverse experiences in patients with AIDS during placebo-controlled clinical trials involved the CNS and were reported by 33% of patients receiving dronabinol capsules. About 25% of patients reported a CNS adverse reaction during the first 2 weeks and about 4% reported such a reaction each week for the next 6 weeks thereafter.
Common Adverse Reactions
The following adverse reactions were reported in clinical trials at an incidence greater than 1%.System Organ Class Adverse Reactions - *
- Actual incidence 3% to 10%
General
Asthenia
Cardiovascular
Palpitations, tachycardia, vasodilation/facial flush
Gastrointestinal
Central Nervous System
Dizziness *, euphoria *, paranoid reaction *, somnolence *, thinking abnormal *, amnesia, anxiety/nervousness, ataxia, confusion, depersonalization, hallucination
Less Common Adverse Reactions
The following adverse reactions were reported in clinical trials at an incidence less than or equal to 1%.System Organ Class Adverse Reactions General
Chills, headache, malaise
Cardiovascular
Hypotension, conjunctival injection [see Clinical Pharmacology (12.2)]
Gastrointestinal
Diarrhea, fecal incontinence, anorexia, hepatic enzyme elevation
Musculoskeletal
Myalgias
Central Nervous System
Depression, nightmares, speech difficulties, tinnitus
Respiratory
Cough, rhinitis, sinusitis
Skin
Flushing, sweating
Sensory
Vision difficulties
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of dronabinol capsules. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General disorders and administration site conditions: Fatigue
Hypersensitivity reactions: Lip swelling, hives, disseminated rash, oral lesions, skin burning, flushing, throat tightness [see Contraindications (4)]
Injury, poisoning, and procedural complications: Fall [see Use in Specific Populations (8.5)]
Nervous system disorders: Seizures [see Warnings and Precautions (5.3)], disorientation, movement disorder, loss of consciousness
Psychiatric disorders: Delirium, insomnia, panic attack
Vascular disorders: Syncope [see Warnings and Precautions (5.2)]
-
7 DRUG INTERACTIONS
7.1 Additive CNS Effects
Additive CNS effects (e.g., dizziness, confusion, sedation, somnolence) may occur when dronabinol capsules are taken concomitantly with drugs that have similar effects on the central nervous system such as CNS depressants [see Warnings and Precautions (5.1)].
7.2 Additive Cardiac Effects
Additive cardiac effects (e.g., hypotension, hypertension, syncope, tachycardia) may occur when dronabinol capsules are taken concomitantly with drugs that have similar effects on the cardiovascular system [see Warnings and Precautions (5.2)].
7.3 Effect of Other Drugs on Dronabinol
Dronabinol is primarily metabolized by CYP2C9 and CYP3A4 enzymes based on published in vitro studies. Inhibitors of these enzymes may increase, while inducers may decrease, the systemic exposure of dronabinol and/or its active metabolite resulting in an increase in dronabinol-related adverse reactions or loss of efficacy of dronabinol capsules.
Monitor for potentially increased dronabinol-related adverse reactions when dronabinol capsules are co-administered with inhibitors of CYP2C9 (e.g., amiodarone, fluconazole) and inhibitors of CYP3A4 enzymes (e.g., ketoconazole, itraconazole, clarithromycin, ritonavir, erythromycin, grapefruit juice).
7.4 Highly Protein-Bound Drugs
Dronabinol is highly bound to plasma proteins, and therefore, might displace and increase the free fraction of other concomitantly administered protein-bound drugs.
Although this displacement has not been confirmed in vivo, monitor patients for increased adverse reactions to narrow therapeutic index drugs that are highly protein-bound (e.g., warfarin, cyclosporine, amphotericin B) when initiating treatment or increasing the dosage of dronabinol capsules.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Dronabinol capsules, a synthetic cannabinoid, may cause fetal harm. Avoid use of dronabinol capsules in pregnant women. Although there is little published data on the use of synthetic cannabinoids during pregnancy, use of cannabis (e.g., marijuana) during pregnancy has been associated with adverse fetal/neonatal outcomes [see Clinical Considerations]. Cannabinoids have been found in the umbilical cord blood from pregnant women who smoke cannabis. In animal reproduction studies, no teratogenicity was reported in mice administered dronabinol (delta-9-THC) at up to 30 times the MRHD (maximum recommended human dose) and up to 5 times the MRHD for patients with AIDS and cancer, respectively. Similar findings were reported in pregnant rats administered dronabinol at up to 5 to 20 times the MRHD and 3 times the MRHD for patients with AIDS and cancer, respectively. Decreased maternal weight gain and number of viable pups and increased fetal mortality and early resorptions were observed in both species at doses which induced maternal toxicity. In rats, maternal administration of dronabinol from pregnancy (implantation) through weaning was associated with maternal toxicity including adverse clinical signs, increased stillbirths and mortality of offspring, and reduced pup bodyweight at 2 and 6 times the MRHD for patients with AIDS, and less than or equal to the MRHD for patients with cancer. No evidence of neurodevelopmental adverse effects was observed in the offspring at doses up to 6 times the MRHD for patients with AIDS, and up to the MRHD for patients with cancer [see Data].The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Published studies suggest that during pregnancy, the use of cannabis, which includes THC, whether for recreational or medicinal purposes, may increase the risk of adverse fetal/neonatal outcomes including fetal growth restriction, low birth weight, preterm birth, small-for-gestational age, admission to the neonatal intensive care unit, and stillbirth. Therefore, use of cannabis during pregnancy should be avoided.Data
Human Data
Delta-9-THC has been measured in the cord blood of some infants whose mothers reported prenatal use of cannabis, suggesting that dronabinol may cross the placenta to the fetus during pregnancy. The effects of delta-9-THC on the fetus are not known.Animal Data
Reproduction studies with dronabinol have been performed in mice at 15 to 450 mg/m 2, equivalent to 1 to 30 times the MRHD of 15 mg/m 2/day in patients with AIDS or 0.2 to 5 times the MRHD of 90 mg/m 2/day in patients with cancer, and in rats at 74 to 295 mg/m 2(equivalent to 5 to 20 times the MRHD of 15 mg/m 2/day in patients with AIDS or 0.8 to 3 times the MRHD of 90 mg/m 2/day in patients with cancer). These studies have revealed no evidence of teratogenicity due to delta-9-THC. At these dronabinol dosages in mice and rats, delta-9-THC decreased maternal weight gain and number of viable pups and increased fetal mortality and early resorptions. Such effects were dose dependent and less apparent at lower doses that produced less maternal toxicity.Review of published literature indicates that the endocannabinoid system plays a role in neurodevelopmental processes such as neurogenesis, migration, and synaptogenesis. Exposure of pregnant rats to delta-9-THC (during and after organogenesis) may modulate these processes to result in abnormal patterns of neuronal connectivity and subsequent cognitive impairments in the offspring. Nonclinical toxicity studies in pregnant rats and newborn pups have shown prenatal exposure to delta-9-THC that resulted in impairment of motor function, alteration in synaptic activity, and interference in cortical projection of neuron development in the offspring. Prenatal exposure has shown effects on cognitive function such as learning, short-and long-term memory, attention, decreased ability to remember task, and ability to discriminate between novel and same objects. Overall, prenatal exposure to delta-9-THC has resulted in significant and long-term changes in brain development, cognition, and behavior in rat offspring.
In a pre- and postnatal development study, female rats were administered dronabinol by oral gavage at doses of 0.5, 5, or 15 mg/kg/day (equivalent to 0.2, 2, and 6 times the MRHD for patients with AIDS and 0.03, 0.33, and 1.0 times the MRHD for patients with cancer, respectively, based on body surface area) from gestation day 6 (implantation) through lactation day 20 (weaning). Maternal toxicity including adverse clinical signs (i.e., decreased motor activity, low carriage, abnormal gait, hunched posture, vocalization to touch, ungroomed coat, mild dehydration, piloerection, and splayed hindlimbs), reduced body weight and body weight gain, and decreased food consumption were observed during the gestation period at 2 and 6 times the MRHD for patients with AIDS and 0.33 and 1.0 times the MRHD for patients with cancer, respectively. At the same doses, reduced pup bodyweight, increased stillbirths, and mortality of offspring were observed. No neurodevelopmental adverse effects (i.e., neurobehavioral function, sensory function, motor activity, learning and memory) were observed in pups at maternal doses up 15 mg/kg/day (6 times the MRHD in patients with AIDS or 1.0 times the MRI-ID in patients with cancer).
8.2 Lactation
Risk Summary
For mothers infected with the Human Immunodeficiency Virus (HIV), the Centers for Disease Control and Prevention recommends that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. Because of the potential for HIV transmission (in HIV-negative infants) and serious adverse reactions in a breastfed infant, instruct mothers not to breastfeed if they are receiving dronabinol capsules.For mothers with nausea and vomiting associated with cancer chemotherapy, there are limited data on the presence of dronabinol in human milk, the effects on the breastfed infant, or the effects on milk production. The reported effects of inhaled cannabis transferred to the breastfeeding infant have been inconsistent and insufficient to establish causality. In rat offspring exposed to dronabinol in utero and during lactation, reduced bodyweight was observed during the preweaning (lactation) stage with maternal administration of dronabinol at 2 times and less than the MRHD for patients with AIDS and cancer, respectively.
Breastfeeding infants should have their weight monitored. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for dronabinol capsules and any potential adverse effects on the breastfed infant from dronabinol capsules or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of dronabinol capsules have not been established in pediatric patients. Pediatric patients may be more sensitive to neurological and psychoactive effects of dronabinol capsules [see Warnings and Precautions (5.1)].
8.5 Geriatric Use
Clinical studies of dronabinol capsules in AIDS and cancer patients did not include the sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Elderly patients may be more sensitive to the neuropsychiatric and postural hypotensive effects of dronabinol capsules [see Warnings and Precautions (5.1, 5.2)].
Elderly patients with dementia are at increased risk for falls as a result of their underlying disease state, which may be exacerbated by the CNS effects of somnolence and dizziness associated with dronabinol capsules [see Warnings and Precautions (5.1)]. These patients should be monitored closely and placed on fall precautions prior to initiating dronabinol capsules therapy. In antiemetic studies, no difference in efficacy was apparent in patients greater than 55 years of age compared to younger patients.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of falls, decreased hepatic, renal, or cardiac function, increased sensitivity to psychoactive effects, and of concomitant disease or other drug therapy [see Dosage and Administration (2.1, 2.2)].
8.6 Effect of CYP2C9 Polymorphism
Published data suggest that systemic clearance of dronabinol may be reduced and concentrations may be increased in the presence of CYP2C9 genetic polymorphism. Monitoring for potentially increased adverse reactions is recommended in patients known to carry genetic variants associated with diminished CYP2C9 function [see Clinical Pharmacology (12.5)].
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Dronabinol capsules contain dronabinol, a Schedule III controlled substance.
9.2 Abuse
Dronabinol capsules contain dronabinol, the main psychoactive component in marijuana. Ingestion of high doses of dronabinol increases the risk of psychiatric adverse reactions if abused or misused, while continued administration can lead to addiction. Psychiatric adverse reactions may include psychosis, hallucinations, depersonalization, mood alteration, and paranoia.
In an open-label study in patients with AIDS who received dronabinol capsules for up to five months, no abuse, diversion or systematic change in personality or social functioning were observed despite the inclusion of a substantial number of patients with a past history of drug abuse.
Patients should be instructed to keep dronabinol capsules in a secure place out of reach of others for whom the medication has not been prescribed.
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use. Physical dependence manifests by drug class-specific withdrawal symptoms after abrupt discontinuation or a significant dose reduction of a drug. The appearance of a withdrawal syndrome when administration of the drug is terminated is the only actual evidence of physical dependence. Physical dependence can develop during chronic therapy with dronabinol capsules, and develops after chronic abuse of marijuana.
A withdrawal syndrome was reported after the abrupt discontinuation of dronabinol in subjects receiving dosages of 210 mg per day for 12 to 16 consecutive days. Within 12 hours after discontinuation, subjects manifested symptoms such as irritability, insomnia, and restlessness. By approximately 24 hours post-dronabinol discontinuation, withdrawal symptoms intensified to include "hot flashes," sweating, rhinorrhea, loose stools, hiccoughs, and anorexia. These withdrawal symptoms gradually dissipated over the next 48 hours.
Electroencephalographic changes consistent with the effects of drug withdrawal (hyperexcitation) were recorded in patients after abrupt dechallenge. Patients also complained of disturbed sleep for several weeks after discontinuing therapy with high dosages of dronabinol.
-
10 OVERDOSAGE
Signs and symptoms of dronabinol overdosage include drowsiness, euphoria, heightened sensory awareness, altered time perception, reddened conjunctiva, dry mouth, tachycardia, memory impairment, depersonalization, mood alteration, urinary retention, reduced bowel motility, decreased motor coordination, lethargy, slurred speech, and postural hypotension. Patients may also experience panic reactions if they have a prior history of nervousness or anxiety, and seizures may occur in patients with existing seizure disorders.
It is not known if dronabinol can be removed by dialysis in cases of overdose.
If over-exposure of dronabinol capsules occurs, call your Poison Control Center at 1-800-222-1222 for current information on the management of poisoning or overdosage.
-
11 DESCRIPTION
Dronabinol is a cannabinoid designated chemically as (6aR,10aR)-6a,7,8,10a-Tetrahydro-6,6,9‑trimethyl-3-pentyl-6H-dibenzo[b,d]-pyran-1-ol. Dronabinol has the following empirical and structural formulas:
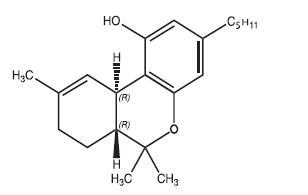
C 21H 30O 2 (molecular weight = 314.46)
Dronabinol, the active ingredient in dronabinol capsules, USP, is synthetic delta-9‑tetrahydrocannabinol (delta-9-THC).
Dronabinol is a light yellow resinous oil that is sticky at room temperature and hardens upon refrigeration.
Dronabinol is insoluble in water and is formulated in sesame oil. It has a pKa of 10.6 and an octanol-water partition coefficient: 6,000:1 at pH 7.
Dronabinol capsule strengths are formulated with the following inactive ingredients: FD&C Yellow No. 6, gelatin, glycerin, purified water, sesame oil, titanium dioxide, iron oxide black 1, shellac glaze 1, isopropyl alcohol 1, n-butyl alcohol 1, propylene glycol 1, and ammonium hydroxide. The 2.5 mg and 5 mg capsules also contain FD&C Blue No. 1 and FD&C Red No. 40.
- 1
- Ingredients in imprint ink
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dronabinol is an orally active cannabinoid which has complex effects on the CNS, including central sympathomimetic activity. Cannabinoid receptors have been discovered in neural tissues. These receptors may play a role in mediating the effects of dronabinol.
12.2 Pharmacodynamics
Effects on the Cardiovascular System
Dronabinol-induced sympathomimetic activity may result in tachycardia and/or conjunctival injection. Its effects on blood pressure are inconsistent, but subjects have experienced orthostatic hypotension and/or syncope upon abrupt standing [see Warnings and Precautions (5.2)].Effects on the Central Nervous System
Dronabinol also demonstrates reversible effects on appetite, mood, cognition, memory, and perception. These phenomena appear to be dose-related, increasing in frequency with higher dosages, and subject to great inter-patient variability. After oral administration, dronabinol has an onset of action of approximately 0.5 to 1 hours and peak effect at 2 to 4 hours. Duration of action for psychoactive effects is 4 to 6 hours, but the appetite stimulant effect of dronabinol may continue for 24 hours or longer after administration.Tachyphylaxis and tolerance develop to some of the pharmacologic effects of dronabinol with chronic use, suggesting an indirect effect on sympathetic neurons. In a study of the pharmacodynamics of chronic dronabinol exposure, healthy male subjects (N = 12) received 210 mg per day of dronabinol capsules, administered orally in divided doses, for 16 days. An initial tachycardia induced by dronabinol was replaced successively by normal sinus rhythm and then bradycardia. A decrease in supine blood pressure, made worse by standing, was also observed initially. These subjects developed tolerance to the cardiovascular and subjective adverse CNS effects of dronabinol within 12 days of treatment initiation.
Tachyphylaxis and tolerance do not appear to develop to the appetite stimulant effect of dronabinol capsules. In clinical studies involving AIDS patients, the appetite stimulant effect of dronabinol capsules was sustained for up to five months at dosages ranging from 2.5 mg to 20 mg per day.
12.3 Pharmacokinetics
Absorption
Dronabinol (delta-9-THC) is almost completely absorbed (90 to 95%) after single oral doses. Due to the combined effects of first pass hepatic metabolism and high lipid solubility, only 10 to 20% of the administered dose reaches the systemic circulation. Concentrations of both parent drug and its major active metabolite (11-hydroxy-delta-9-THC) peak at approximately 0.5 to 4 hours after oral dosing and decline over several days.The pharmacokinetics of dronabinol after single doses (2.5, 5, and 10 mg) and multiple doses (2.5, 5, and 10 mg given twice a day) have been studied in healthy subjects.
Summary of Multiple-Dose Pharmacokinetic Parameters of Dronabinol in Healthy Subjects (n=34; 20 to 45 years) under Fasted Conditions Mean (SD) PK Parameter Values Twice Daily Dose C max ng/mL Median T max (range), hr AUC (0-12) ng∙hr/mL C max: maximum observed plasma concentration; T max: time to maximum observed plasma concentration; AUC (0-12): area under the plasma concentration-time curve from 0 to 12 hours. 2.5 mg
1.32 (0.62)
1.00 (0.50-4.00)
2.88 (1.57)
5 mg
2.96 (1.81)
2.50 (0.50-4.00)
6.16 (1.85)
10 mg
7.88 (4.54)
1.50 (0.50-3.50)
15.2 (5.52)
A slight increase in dose proportionality on mean C max and AUC (0-12) of dronabinol was observed with increasing dose over the dose range studied.
Effect of Food:
In a published study, the effect of food on the pharmacokinetics of dronabinol was studied by concomitant dosing of dronabinol capsules with a high-fat (59 grams of fat, approximately 50% of total caloric content of the meal), high calorie meal (approximately 950 calories). An appreciable food effect was observed, resulting in a 4-hour delay in mean T max and 2.9-fold increase in total exposure (AUC inf), but C max was not significantly changed [see Dosage and Administration (2.2)].Distribution
Dronabinol has an apparent volume of distribution of approximately 10 L/kg, because of its lipid solubility. The plasma protein binding of dronabinol and its metabolites is approximately 97% [see Drug Interactions (7.4)].Elimination
The pharmacokinetics of dronabinol can be described using a two-compartment model with an initial (alpha) half-life of about 4 hours and a terminal (beta) half-life of 25 to 36 hours. Values for clearance average about 0.2 L/kg-hr, but are highly variable due to the complexity of cannabinoid distribution.Metabolism
Dronabinol undergoes extensive first-pass hepatic metabolism, primarily by hydroxylation, yielding both active and inactive metabolites. Dronabinol and its principal active metabolite, 11‑hydroxy-delta-9-THC, are present in approximately equal concentrations in plasma. Published in vitro data indicates that CYP2C9 and CYP3A4 are the primary enzymes in the metabolism of dronabinol. CYP2C9 appears to be the enzyme responsible for the formation of the primary active metabolite [see Clinical Pharmacology (12.5)].Excretion
Dronabinol and its biotransformation products are excreted in both feces and urine. Biliary excretion is the major route of elimination with about half of a radiolabeled oral dose being recovered from the feces within 72 hours as contrasted with 10 to 15% recovered from urine. Less than 5% of an oral dose is recovered unchanged in the feces.Due to its re-distribution, dronabinol and its metabolites may be excreted at low levels for prolonged periods of time. Following single dose administration, low levels of dronabinol metabolites have been detected for more than 5 weeks in the urine and feces.
In a study of dronabinol capsules involving AIDS patients, urinary cannabinoid/creatinine concentration ratios were studied bi-weekly over a six-week period. The urinary cannabinoid/creatinine ratio was closely correlated with dose. No increase in the cannabinoid/creatinine ratio was observed after the first two weeks of treatment, indicating that steady-state cannabinoid levels had been reached. This conclusion is consistent with predictions based on the observed terminal half-life of dronabinol.
Drug Interaction Studies
Formal drug-drug interaction studies have not been conducted with dronabinol.The enzyme inhibition and induction potential of dronabinol and its active metabolite are not completely understood.
Published data showed an increase in the elimination half-life of pentobarbital by 4 hours when concomitantly dosed with dronabinol [see Warnings and Precautions (5.1)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In 2-year carcinogenicity studies, there was no evidence of carcinogenicity in rats at doses up to 50 mg/kg/day dronabinol (approximately 20 times the MRHD in AIDS patients on a body surface area basis) or in mice at doses up to 500 mg/kg/day (approximately 100 times the MRHD in AIDS patients on a body surface area basis).
Dronabinol was not genotoxic in the Ames tests, the in vitro chromosomal aberration test in Chinese hamster ovary cells, and the in vivo mouse micronucleus test. However, dronabinol produced a weak positive response in a sister chromatid exchange test in Chinese hamster ovary cells.
In a long-term study (77 days) in rats, oral administration of dronabinol at doses of 30 to 150 mg/m 2, equivalent to 2 to 10 times the MRHD of 15 mg/m 2/day in AIDS patients or 0.3 to 1.5 times the MRHD of 90 mg/m 2/day in cancer patients, reduced ventral prostate, seminal vesicle and epididymal weights and caused a decrease in seminal fluid volume. Decreases in spermatogenesis, number of developing germ cells, and number of Leydig cells in the testis were also observed. However, sperm count, mating success, and testosterone levels were not affected. The significance of these animal findings in humans is not known.
-
14 CLINICAL STUDIES
The effectiveness of dronabinol capsules has been established based on studies for the treatment of anorexia associated with weight loss in patients with AIDS and nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments.
14.1 Appetite Stimulation
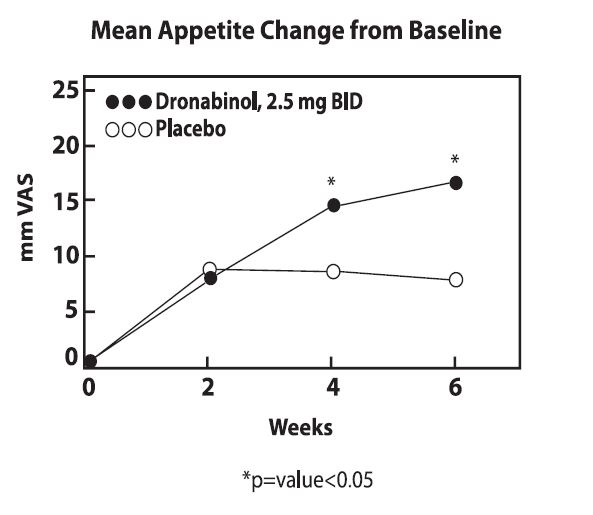
The appetite stimulant effect of dronabinol capsules in the treatment of AIDS-related anorexia associated with weight loss was studied in a randomized, double-blind, placebo-controlled study involving 139 patients. The initial dosage of dronabinol capsules in all patients was 5 mg/day, administered in doses of 2.5 mg one hour before lunch and one hour before dinner. In pilot studies, early morning administration of dronabinol capsules appeared to have been associated with an increased frequency of adverse experiences, as compared to dosing later in the day. The effect of dronabinol capsules on appetite, weight, mood, and nausea was measured at scheduled intervals during the six-week treatment period. Side effects (feeling high, dizziness, confusion, somnolence) occurred in 13 of 72 patients (18%) at this dosage level and the dosage was reduced to 2.5 mg/day, administered as a single dose at supper or bedtime.
Of the 112 patients that completed at least 2 visits in the randomized, double-blind, placebo-controlled study, 99 patients had appetite data at 4-weeks (50 received dronabinol capsules and 49 received placebo) and 91 patients had appetite data at 6-weeks (46 received dronabinol capsules and 45 received placebo). A statistically significant difference between dronabinol capsules and placebo was seen in appetite as measured by the visual analog scale at weeks 4 and 6 (see figure). Trends toward improved body weight and mood, and decreases in nausea were also seen.
After completing the 6-week study, patients were allowed to continue treatment with dronabinol capsules in an open-label study, in which there was a sustained improvement in appetite.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
DRONABINOL CAPSULES, USP are supplied as:
2.5 mg oblong opaque cream capsules (Identified as RP 867).
Unit dose packages of 30 (3 x 10) NDC 60687-375-21.
Unit dose packages of 100 (10 x 10) NDC 60687-375-01.5 mg oblong opaque brown capsules (Identified as RP 868).
Unit dose packages of 20 (2 x 10) NDC 60687-386-94.
Unit dose packages of 30 (3 x 10) NDC 60687-386-21.Storage Conditions
Dronabinol Capsules, USP should be stored in a refrigerator 2° to 8°C (36° to 46°F). Protect from freezing.FOR YOUR PROTECTION: Do not use if blister is torn or broken.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Neuropsychiatric Adverse Reactions[see Warnings and Precautions (5.1)]
- Advise patients that psychiatric adverse reactions may occur, especially in patients with a past psychiatric history or in those receiving other drugs also associated with psychiatric effects, and to report to their healthcare provider any new or worsening psychiatric symptoms.
- Advise patients, especially elderly patients, that cognitive impairment or an altered mental state may also occur during treatment with dronabinol capsules and to report to their healthcare provider if they develop signs or symptoms of cognitive impairment.
- Advise patients not to operate motor vehicles or other dangerous machinery until they are reasonably certain that dronabinol capsules do not affect them adversely. Alert patients to the potential for additive central nervous system depression if dronabinol capsules are used concomitantly with alcohol or other CNS depressants such as benzodiazepines and barbiturates.
Hemodynamic Instability
Advise patients, especially those with cardiac disorders, to report to their healthcare provider if they experience any signs or symptoms of hemodynamic instability, including hypotension, hypertension, syncope, or tachycardia, especially after initiating or increasing the dosage of dronabinol capsules [see Warnings and Precautions (5.2)].Seizures
Advise patients to discontinue dronabinol capsules and contact a healthcare provider immediately if they experience a seizure [see Warnings and Precautions (5.3)].Multiple Substance Abuse
Inform patients with a history of substance abuse or dependence, including marijuana or alcohol, that they may be more likely to abuse dronabinol capsules. Advise patients to report to their healthcare provider if they develop abuse behaviors or conditions [see Warnings and Precautions (5.4)].Paradoxical Nausea, Vomiting, or Abdominal Pain
Advise patients to report worsening nausea, vomiting or abdominal pain to their healthcare provider [see Warnings and Precautions (5.5)].Pregnancy
Advise pregnant women of the potential risk to a fetus and to avoid use of dronabinol capsules during pregnancy [see Use in Specific Populations (8.1)].Lactation
- Advise HIV infected women with anorexia associated with weight loss, not to breastfeed because HIV can be passed to the baby through the breast milk.
- Advise women with nausea and vomiting associated with cancer chemotherapy that breastfeeding infants should have their weight monitored [see Use in Specific Populations (8.2)].
-
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see How Supplied section) contain drug product from Rhodes Pharmaceuticals as follows:
(2.5 mg / 30 UD) NDC 60687-375-21 packaged from NDC 42858-867
(2.5 mg / 100 UD) NDC 60687-375-01 packaged from NDC 42858-867
(5 mg / 20 UD) NDC 60687-386-94 packaged from NDC 42858-868
(5 mg / 30 UD) NDC 60687-386-21 packaged from NDC 42858-868Distributed by:
American Health Packaging
Columbus, OH 432178437501/0324
-
PATIENT INFORMATION
- *
- Ingredients in imprint ink
8437501/0324
DRONABINOL (droe nab' i nol) CAPSULES, USP
for oral use, CIII
What is the most important information I should know about dronabinol capsules?
Dronabinol capsules can cause serious side effects, including:
- Worsening mental (psychiatric) symptoms. Psychiatric symptoms can worsen in people who have mania, depression, or schizophrenia and who take dronabinol capsules. Dronabinol capsules taken with medicines that cause psychiatric symptoms can worsen psychiatric symptoms. Elderly people who take dronabinol capsules may have a greater risk of having psychiatric symptoms. Tell your doctor if you have new or worsening mood symptoms, including symptoms of mania, depression, or schizophrenia.
- Problems thinking clearly. Tell your doctor if you have trouble remembering things, concentrating, have increased sleepiness, or confusion. Elderly people may have a greater risk of having problems thinking clearly.
- Changes in your blood pressure. Dronabinol capsules may increase or decrease your blood pressure, especially when you start taking dronabinol capsules or when your dose is changed. Tell your doctor if you have signs or symptoms of changes in your blood pressure including: headaches, vision problems, dizziness, feeling lightheaded, fainting, or a fast heartbeat. Elderly people, especially those with dementia, and people with heart problems may have an increased risk of changes in blood pressure and an increased risk of falls.
What are dronabinol capsules?
- Dronabinol capsules are a prescription medicine used in adults to treat:
- loss of appetite (anorexia) in people with AIDS (Acquired Immune Deficiency Syndrome) who have lost weight.
- nausea and vomiting caused by anti-cancer medicine (chemotherapy) in people whose nausea and vomiting have not improved with usual anti-nausea medicines.
Dronabinol capsules are a controlled substance (CIII) because it contains dronabinol, which can be a target for people who abuse prescription medicines or street drugs. Keep your dronabinol capsules in a safe place to protect them from theft. Never give your dronabinol capsules to anyone else because they may cause death or harm them. Selling or giving away this medicine is against the law.
It is not known if dronabinol capsules are safe and effective in children.
Do not take dronabinol capsules if you:
- had an allergic reaction to dronabinol. Signs and symptoms of an allergic reaction to dronabinol include: swelling of the lips, hives, a rash over your whole body, mouth sores, skin burning, flushing, and throat tightness.
- had an allergic reaction to sesame oil.
Before taking dronabinol capsules, tell your doctor about all of your medical conditions, including if you:
- have or had heart problems.
- have or had problems with drug abuse or dependence.
- have or had problems with alcohol abuse or dependence.
- have or had mental health problems including mania, depression, or schizophrenia.
- have had a seizure or have a medical condition that may increase your risk of having a seizure.
- are pregnant or plan to become pregnant. Dronabinol capsules may harm your unborn baby. Avoid the use of dronabinol capsules if you are pregnant.
- are breastfeeding or plan to breastfeed. It is not known if dronabinol passes into your breast milk. Talk to your
- doctor about the best way to feed your baby if you take dronabinol capsules. The Centers for Disease Control and Prevention recommends that mothers with HIV not breastfeed because they can pass the HIV through their breast milk to the baby. If you are being treated for nausea and vomiting caused by anti-cancer medicine and you breastfeed while taking dronabinol capsules, your doctor should check the weight of your baby regularly.
Tell your doctor about all the medicines you take or have taken in the last 14 days, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Dronabinol capsules and certain other medicines can affect each other, causing serious side effects.
How should I take Dronabinol capsules?
- Take dronabinol capsules exactly as your doctor tells you to. Your doctor may change your dose after seeing how it affects you. Do not change your dose unless your doctor tells you to change it.
- If you are an adult with AIDS with loss of appetite and weight loss:
- Dronabinol capsules are usually taken 2 times each day, 1 hour before lunch and 1 hour before dinner. If you are elderly or unable to tolerate this dose of dronabinol capsules, your doctor may prescribe dronabinol capsules to be taken 1 time each day, 1 hour before dinner or bedtime to reduce your chance of having nervous system problems.
- If you are an adult with nausea and vomiting caused by anti-cancer medicine:
- Dronabinol capsules are usually taken 1 to 3 hours before your chemotherapy treatment and then every 2 to 4 hours after chemotherapy for up to 4 to 6 doses each day. If you are elderly, your doctor may prescribe dronabinol capsules to be taken 1 to 3 hours before your chemotherapy, 1 time each day to reduce your chance of having nervous system problems.
- Take your first dose of dronabinol capsules on an empty stomach at least 30 minutes before eating. After your first dose of dronabinol capsules, you can take dronabinol capsules with or without food. Always take your dose at the same time in relation to your mealtimes for each chemotherapy treatment.
- If you take too much dronabinol capsules, call your Poison Control Center at 1-800-222-1222 right away.
What should I avoid while taking dronabinol capsules?
- Do not drive, operate machinery, or do other dangerous activities until you know how dronabinol capsules affects you. Dronabinol capsules taken with other medicines that cause dizziness, confusion, and sleepiness may make these symptoms worse.
What are the possible side effects of dronabinol capsules?
Dronabinol capsules may cause serious side effects, including:- See "What is the most important information I should know about dronabinol capsules?"
- Seizures. Dronabinol capsules may increase your risk of seizures. Stop taking dronabinol capsules and call your doctor and get medical care right away if you have a seizure during treatment with dronabinol capsules.
- Drug and alcohol abuse. You may have an increased risk of abusing dronabinol capsules if you have a history of drug or alcohol abuse or dependence, including marijuana. Tell your doctor if you develop abuse behaviors such as increased irritability, nervousness, restlessness or want more or higher doses of dronabinol capsules during your treatment.
- Nausea, vomiting, or stomach-area (abdominal) pain. Tell your doctor if you have nausea, vomiting, or abdominal pain or if your nausea, vomiting, or abdominal pain gets worse during treatment with dronabinol capsules.
The most common side effects of dronabinol capsules include:
- dizziness
- feeling extremely happy (euphoria)
- overly suspicious or feeling people want to harm you (paranoid reaction)
- sleepiness
- abnormal thoughts
- stomach-area (abdominal) pain
- nausea
- vomiting
These are not all the possible side effects of dronabinol capsules. Tell your doctor if you have any side effect that bothers you or does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store dronabinol capsules?
- Store dronabinol capsules in a cool place such as in a refrigerator, at a temperature between 36° to 46°F (2° to 8°C).
- Do not freeze dronabinol capsules.
Keep dronabinol capsules and all medicines out of the reach of children.
General information about the safe and effective use of dronabinol capsules
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use dronabinol capsules for a condition for which it was not prescribed. Do not give dronabinol capsules to other people, even if they have the same symptoms that you have. It may harm them. You can ask your doctor or pharmacist for information about dronabinol capsules that is written for health professionals.
What are the ingredients in dronabinol capsules?
Active ingredient: dronabinol
Inactive ingredients: FD&C Yellow No. 6, gelatin, glycerin, purified water, sesame oil, titanium dioxide, iron oxide black *, shellac glaze *, isopropyl alcohol *, n-butyl alcohol *, propylene glycol *, and ammonium hydroxide. The 2.5 mg and 5 mg capsules also contain FD&C Blue No. 1 and FD&C Red No. 40.
For more information about the drug product, call Rhodes Pharmaceuticals L.P. at 1-888-827-0616
For more information about the packaging or labeling, call American Health Packaging at 1‐800‐707‐4621.This Patient Information has been approved by the U.S. Food and Drug Administration.
Distributed by:
American Health Packaging
Columbus, OH 432178437501/0324
-
Package/Label Display Panel – Carton – 2.5 mg – 30 UD

NDC 60687- 375-21
Dronabinol
Capsules, USP CIII2.5 mg
30 Capsules (3 x 10) Rx Only
PHARMACIST: Dispense with Medication Guide to each patient.
Each Capsule Contains:
Dronabinol, USP...................................................................... 2.5 mgUsual Dosage: See full prescribing information.
Store in a refrigerator 2° to 8°C (36° to 46°F)
Protect from freezing.FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
NDC # 42858-867, Rhodes Pharmaceuticals.Distributed by: American Health Packaging, Columbus, Ohio 43217
737521
0437521/0324 -
Package/Label Display Panel – Carton – 2.5 mg – 100 UD

NDC 60687- 375-01
Dronabinol
Capsules, USP CIII2.5 mg
100 Capsules (10 x 10) Rx Only
PHARMACIST: Dispense with the accompanying Medication
Guide to each patient.Each Capsule Contains:
Dronabinol, USP................................................................2.5 mgUsual Dosage: See package insert for full prescribing
information.Store in a refrigerator 2° to 8°C (36° to 46°F).
Protect from freezing.Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or
broken.The drug product contained in this package is from
NDC # 42858-867, Rhodes Pharmaceuticals L.P.Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217737501
0437501/0918 - Package/Label Display Panel – Blister – 2.5 mg
-
Package/Label Display Panel – Carton – 5 mg – 20 UD

NDC 60687- 386-94
Dronabinol
Capsules, USP CIII5 mg
20 Capsules (2 x 10) Rx Only
PHARMACIST: Dispense with Medication Guide to each patient.
Each Capsule Contains:
Dronabinol, USP......................................................................... 5 mgUsual Dosage: See full prescribing information.
Store in a refrigerator 2° to 8°C (36° to 46°F)
Protect from freezing.FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
NDC # 42858-868, Rhodes Pharmaceuticals.Distributed by: American Health Packaging, Columbus, Ohio 43217
738694
0438694/0324A -
Package/Label Display Panel – Carton – 5 mg – 30 UD

NDC 60687- 386-21
Dronabinol
Capsules, USP CIII5 mg
30 Capsules (3 x 10) Rx Only
PHARMACIST: Dispense with the accompanying Medication
Guide to each patient.Each Capsule Contains:
Dronabinol, USP............................................................... 5 mgUsual Dosage: See package insert for full prescribing
information.Store in a refrigerator 2° to 8°C (36° to 46°F).
Protect from freezing.Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
NDC # 42858-868, Rhodes Pharmaceuticals L.P.Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217738621
0438621/0918 - Package/Label Display Panel – Blister – 5 mg
-
INGREDIENTS AND APPEARANCE
DRONABINOL
dronabinol capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60687-375(NDC:42858-867) Route of Administration ORAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DRONABINOL (UNII: 7J8897W37S) (DRONABINOL - UNII:7J8897W37S) DRONABINOL 2.5 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SESAME OIL (UNII: QX10HYY4QV) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color white (Opaque cream capsules) Score no score Shape CAPSULE (oblong softgel) Size 12mm Flavor Imprint Code RP;867 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60687-375-21 30 in 1 CARTON 03/31/2024 1 NDC:60687-375-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:60687-375-01 100 in 1 BOX, UNIT-DOSE 07/16/2018 09/30/2025 2 NDC:60687-375-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078292 07/16/2018 DRONABINOL
dronabinol capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60687-386(NDC:42858-868) Route of Administration ORAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DRONABINOL (UNII: 7J8897W37S) (DRONABINOL - UNII:7J8897W37S) DRONABINOL 5 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SESAME OIL (UNII: QX10HYY4QV) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color brown (Opaque brown capsules) Score no score Shape CAPSULE (oblong softgel) Size 12mm Flavor Imprint Code RP;868 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60687-386-94 20 in 1 CARTON 03/31/2024 1 NDC:60687-386-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:60687-386-21 30 in 1 BOX, UNIT-DOSE 08/09/2018 10/31/2025 2 NDC:60687-386-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078292 08/09/2018 Labeler - American Health Packaging (929561009) Establishment Name Address ID/FEI Business Operations American Health Packaging 929561009 repack(60687-375, 60687-386)








