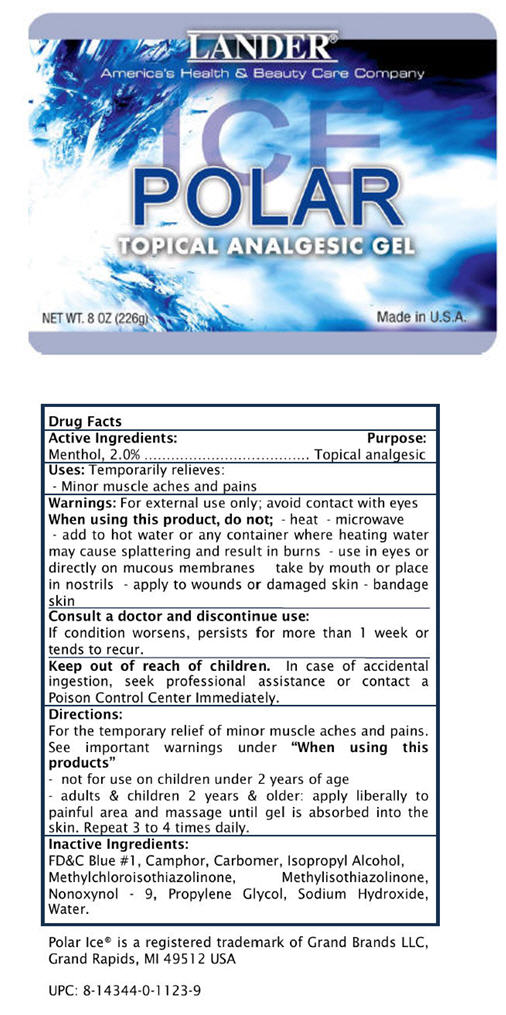
Label: LANDER POLAR ICE- menthol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 20890-0020-1 - Packager: Abaco Partners LLC DBA Surefil
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 18, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only; avoid contact with eyes
When using this product, do not;
- -
- heat
- -
- microwave
- -
- add to hot water or any container where heating water may cause splattering and result in burns
- -
- use in eyes or directly on mucous membranes
- take by mouth or place in nostrils
- -
- apply to wounds or damaged skin
- -
- bandage skin
-
Directions
For the temporary relief of minor muscle aches and pains. See important warnings under "When using this products"
- -
- not for use on children under 2 years of age
- -
- adults & children 2 years & older: apply liberally to painful area and massage until gel is absorbed into the skin. Repeat 3 to 4 times daily.
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 226g Container
-
INGREDIENTS AND APPEARANCE
LANDER POLAR ICE
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:20890-0020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Menthol (UNII: L7T10EIP3A) (Menthol - UNII:L7T10EIP3A) Menthol 20 mg in 1 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Propylene Glycol (UNII: 6DC9Q167V3) Camphor (SYNTHETIC) (UNII: 5TJD82A1ET) Nonoxynol-9 (UNII: 48Q180SH9T) Isopropyl Alcohol (UNII: ND2M416302) Carbomer Homopolymer Type C (UNII: 4Q93RCW27E) Sodium Hydroxide (UNII: 55X04QC32I) FD&C Blue NO. 1 (UNII: H3R47K3TBD) Methylchloroisothiazolinone (UNII: DEL7T5QRPN) Methylisothiazolinone (UNII: 229D0E1QFA) Product Characteristics Color BLUE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:20890-0020-1 226 g in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part356 12/01/2010 Labeler - Abaco Partners LLC DBA Surefil (964809417) Establishment Name Address ID/FEI Business Operations Anicare Pharmaceutical Pvt. 916837425 MANUFACTURE