Label: ADVANCED FIRMING AND ANTI-WRINKLE DAY SPF 18 EQB- avobenzone 2% octisalate 5% octocrylene 5% cream
- NDC Code(s): 79903-206-02
- Packager: Walmart
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
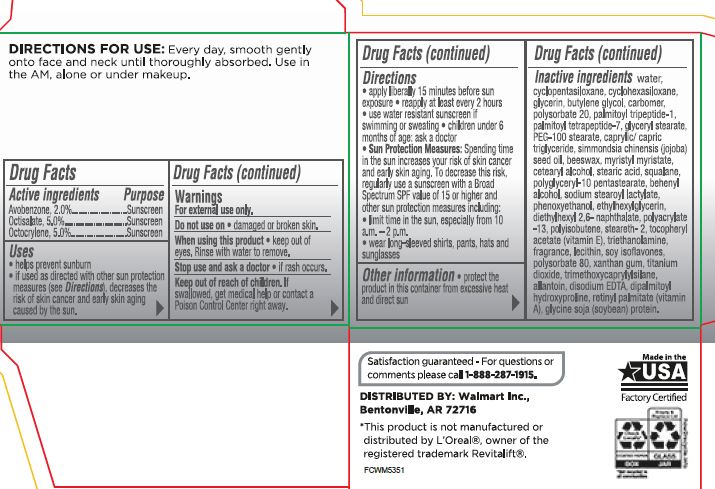
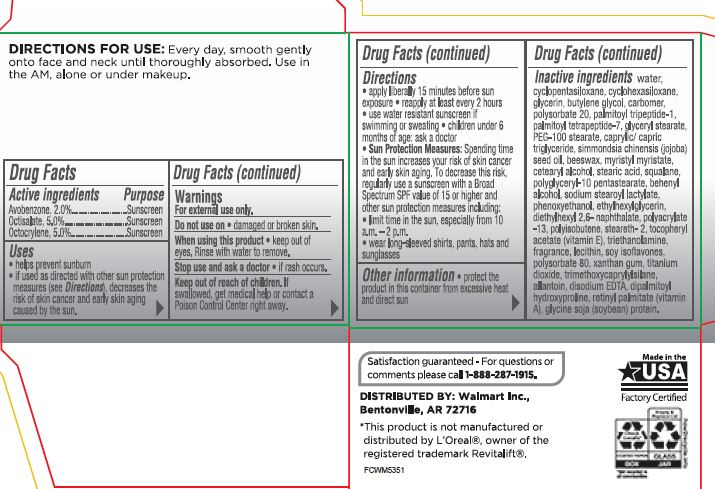
- Active ingredients Purpose
- PURPOSE
- Uses
- Warnings For external use only
- Do not use on
- When using this product
- Stop use and ask a doctor
- Keep out of reach of children. If swallowed,get medical help orcontact a Poison Control Center right away
-
Directions:
• apply liberal 15 minutes before sun exposure• reapply at least every 2 hours •use a water resistant sunscreen if swimmingor sweating•children under 6 months of age: ask a doctor• Sun Protection Measures:Spending time in the sun increase your risk of skin cancer and early skin aging. To decrease this risk. regularly usea sunscreen wrtha Broad Spectrum SPF value of15 or higher and other suncrin protection measures including:
• limrt time in the sun, especial from 10a.m. -2 p.rn.•wear long-sleeve shorts , pants, hats and sunglasses - Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ADVANCED FIRMING AND ANTI-WRINKLE DAY SPF 18 EQB
avobenzone 2% octisalate 5% octocrylene 5% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-206 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength OCTOCRYLENE (UNII: 5A68WGF6WM) 5 g in 100 g WATER (UNII: 059QF0KO0R) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) CYCLOMETHICONE 6 (UNII: XHK3U310BA) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CARBOMER 940 (UNII: 4Q93RCW27E) POLYSORBATE 20 (UNII: 7T1F30V5YH) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) PEG-100 STEARATE (UNII: YD01N1999R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) JOJOBA OIL (UNII: 724GKU717M) YELLOW WAX (UNII: 2ZA36H0S2V) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) STEARIC ACID (UNII: 4ELV7Z65AP) SQUALANE (UNII: GW89575KF9) POLYGLYCERYL-10 PENTASTEARATE (UNII: PMX5872701) SODIUM STEAROYL LACTYLATE (UNII: IN99IT31LN) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) DIETHYLHEXYL 2,6-NAPHTHALATE (UNII: I0DQJ7YGXM) POLYACRYLATE-13 (UNII: FS2D4T67EA) TROLAMINE (UNII: 9O3K93S3TK) POLYISOBUTYLENE (2300 MW) (UNII: DSQ2V1DD1K) STEARETH-2 (UNII: V56DFE46J5) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SOY ISOFLAVONES (UNII: 71B37NR06D) POLYSORBATE 80 (UNII: 6OZP39ZG8H) XANTHAN GUM (UNII: TTV12P4NEE) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIMETHOXYCAPRYLYLSILANE (UNII: FZ07E4LW2M) ALLANTOIN (UNII: 344S277G0Z) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SOY PROTEIN (UNII: R44IWB3RN5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-206-02 1 in 1 CARTON 04/01/2024 1 48 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 Labeler - Walmart (051957769) Registrant - Bridgeview Investments LLC (035014854) Establishment Name Address ID/FEI Business Operations Derma Care research labs. LLC 116817470 manufacture(79903-206)