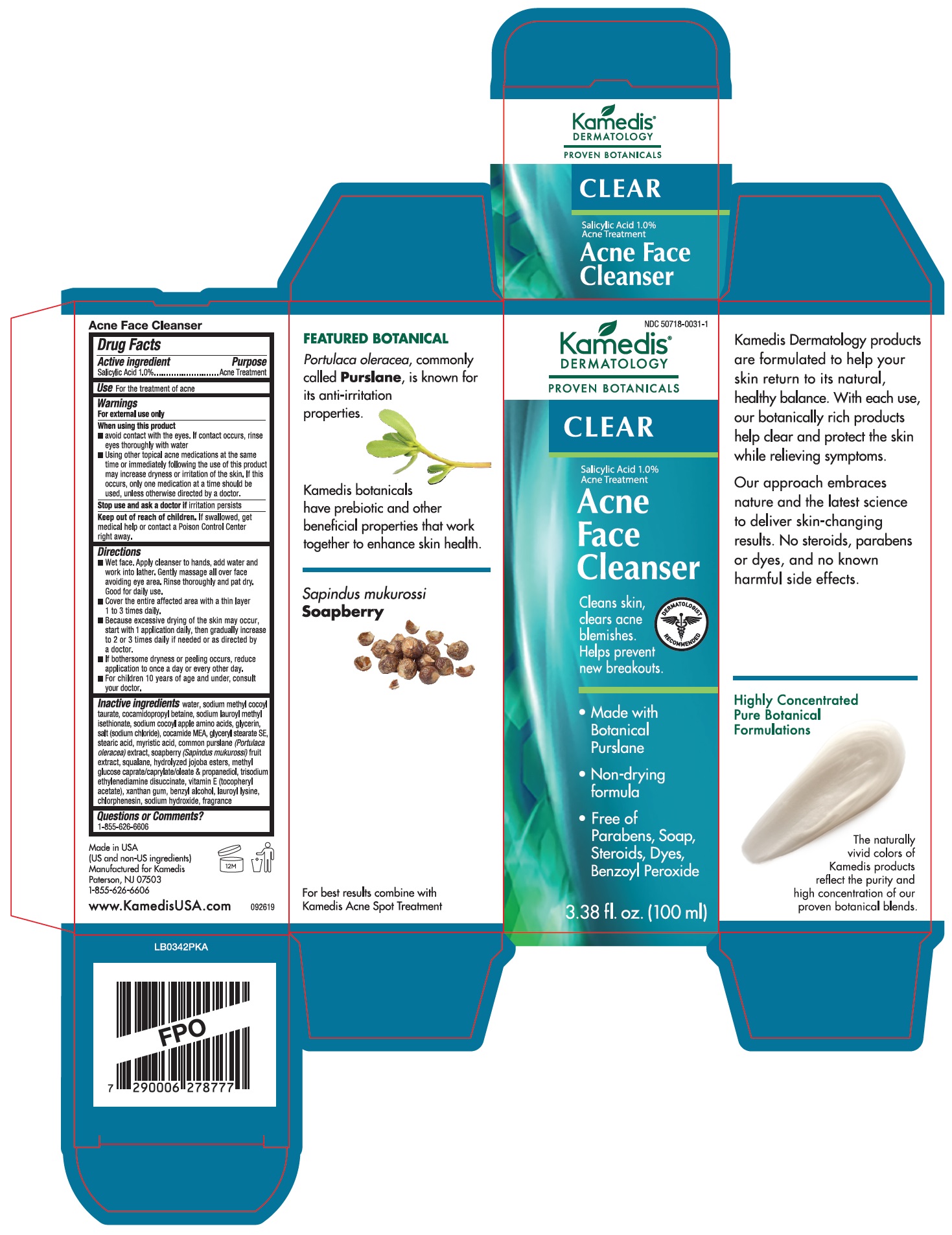
Label: CLEAR ACNE FACE CLEANSER- acne face cleanser liquid
- NDC Code(s): 50718-0031-1
- Packager: Kamedis
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
When using this product
- avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water
- using other topical acne medications at the same time or immediately following the use of this product may increase dryness or irritation of the skin. If this occurs, only one medication at a time should be used, unless otherwise directed by a doctor.
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wet face. Apply cleanser to hands, add water and work into lather. Gently massage all over face avoiding eye area. Rinse thoroughly and pat dry. Good for daily use.
- Cover the entire affected area with a thin layer 1 to 3 times daily.
- Because excessive drying of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- For children under 10 years of age and under, consult your doctor.
Inactive Ingredients
water, sodium methyl cocoyl taurate, cocamidopropyl betaine, sodium lauroyl methyl isethionate, sodium cocoyl apple amino acids, glycerin, salt (sodium chloride), cocamide MEA, glyceryl stearate SE, stearic acid, myristic acid, common purslane (Portulaca oleracea) extract, soapberry (Sapindus mukurossi) fruit extract, squalane, hydrolyzed jojoba esters, methyl glucose caprate/caprylate/oleate & propanediol, trisodium ethylenediamine disuccinate, vitamin E (tocopheryl acetate), xanthan gum, benzyl alcohol, lauroyl lysine, chlorphenesin, sodium hydroxide, fragrance
- SPL UNCLASSIFIED SECTION
- Carton label
-
INGREDIENTS AND APPEARANCE
CLEAR ACNE FACE CLEANSER
acne face cleanser liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50718-0031 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 14.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) PURSLANE (UNII: M6S840WXG5) CHLORPHENESIN (UNII: I670DAL4SZ) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM HYDROXIDE (UNII: 55X04QC32I) SQUALANE (UNII: GW89575KF9) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) COCO MONOETHANOLAMIDE (UNII: C80684146D) LAUROYL LYSINE (UNII: 113171Q70B) MYRISTIC ACID (UNII: 0I3V7S25AW) SODIUM CHLORIDE (UNII: 451W47IQ8X) SAPINDUS MUKOROSSI FRUIT (UNII: 66H9NW427Y) SODIUM LAUROYL METHYL ISETHIONATE (UNII: II6VCD3S6R) SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) STEARIC ACID (UNII: 4ELV7Z65AP) EDETATE SODIUM (UNII: MP1J8420LU) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYDROLYZED JOJOBA ESTERS (ACID FORM) (UNII: UDR641JW8W) SODIUM COCOYL APPLE AMINO ACIDS (UNII: EB3U1PA61T) METHYL GLUCOSE (UNII: QCF122NF3R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50718-0031-1 1 in 1 CARTON 01/01/2018 1 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M006 01/01/2018 Labeler - Kamedis (080311300) Establishment Name Address ID/FEI Business Operations Biogenesis Inc. 069117328 manufacture(50718-0031)