Label: ARAMARK PAIN FREE- acetaminophen, aspirin, caffeine, salicylamide tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 81238-0242-1, 81238-0242-2 - Packager: Western First Aid Safety DBA Aramark
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 3, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings:
• Reye’s Syndrome: Children and teenagers should not
use this medicine for chicken pox or flu symptoms
before a doctor is consulted about Reye’s Syndrome,
a rare but serious illness reported to be assocated
with aspirin.
• Allergy Alert - Do not use:
• if you are allergic to aspirin or any other salicylate
• if you have ever had an allergic reaction to any
other pain reliever/fever reducer
• Liver Warning: This product contains acetaminophen.
Severe liver damage may occur if you take:
• more than 8 tablets in 24 hours
• with other drugs containing acetaminophen
(prescription or nonprescription)
• Ask a doctor or pharmacist before using with
other drugs if you are not sure.
• 3 or more alcoholic drinks every day while using
this product.• Stomach Bleeding Warning: This product contains
nonsteroidal anti-inflammatory drugs (NSAIDs),
which may cause stomach bleeding. The chance is
higher if you:
• are age 60 or older
• have had stomach ulcers or bleeding problems
• take a blood thinning (anticoagulant) or steroid
drug
• take other drugs containing an NSAID (aspirin,
ibuprofen, naproxen, or others)
• take more or for a longer time than directed
• Do not use:
• with any other pain reliever/fever reducer
• for pain for more than 10 days or for fever for
more than 3 days unless directed by a doctor
• with any other product containing
acetaminophen -
ASK DOCTOR
Ask a doctor before using if you have:
• upset stomach or stomach pain • ulcers
• bleeding problems • high blood pressure • heart or
kidney disease • taken a diuretic • reached age 60
or older
Ask a doctor or pharmacist before use if you are:
• taking any other drug containing an NSAID
(prescription or nonprescription)
• taking a blood thinning (anticoagulant) or
steroid drug
When using this product do not exceed the
recommended dose. - STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
-
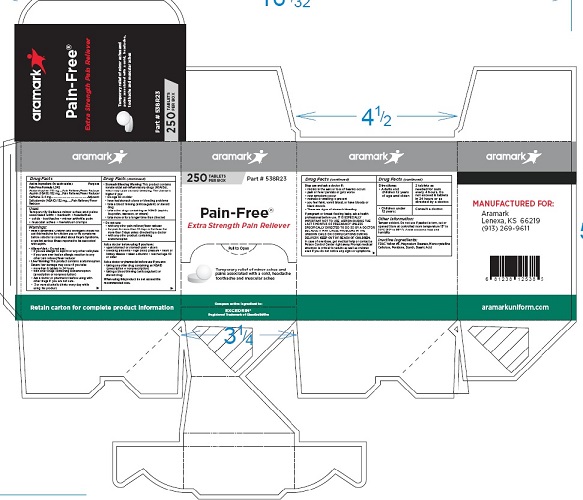
Product Package
aramark
Pain-Free®
Extra Strength Pain Reliever
Temporary relief of minor
aches and pains associated with
a cold, headache, toothache and
muscular achesPart # 537R23
100 TABLETS
PER BOX
Compare active ingredient to:
EXCEDRIN®
Registered Trademark of GlaxoSmithKlineRetain carton for complete product information
MANUFACTURED FOR:
Aramark
Lenexa, KS 66219
(913) 269-9611aramarkuniform.com
100 Tablet Box

250 Tablet Box
2-Tablet packet

res
-
INGREDIENTS AND APPEARANCE
ARAMARK PAIN FREE
acetaminophen, aspirin, caffeine, salicylamide tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81238-0242 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 110 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 162 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 32.4 mg SALICYLAMIDE (UNII: EM8BM710ZC) (SALICYLAMIDE - UNII:EM8BM710ZC) SALICYLAMIDE 152 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color orange Score no score Shape ROUND Size 11mm Flavor Imprint Code FR2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81238-0242-1 50 in 1 BOX 05/07/2021 1 2 in 1 PACKET; Type 0: Not a Combination Product 2 NDC:81238-0242-2 125 in 1 BOX 05/07/2021 2 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 05/07/2021 Labeler - Western First Aid Safety DBA Aramark (043861524) Registrant - Western First Aid Safety DBA Aramark (043861524)