Label: SOLUTIONKITS ENROFLOXACIN- enrofloxacin kit
- NDC Code(s): 46144-605-01
- Packager: API Solutions
- Category: BULK INGREDIENT - ANIMAL DRUG
- DEA Schedule: None
- Marketing Status: Bulk Ingredient For Animal Drug Compounding
Drug Label Information
Updated January 3, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
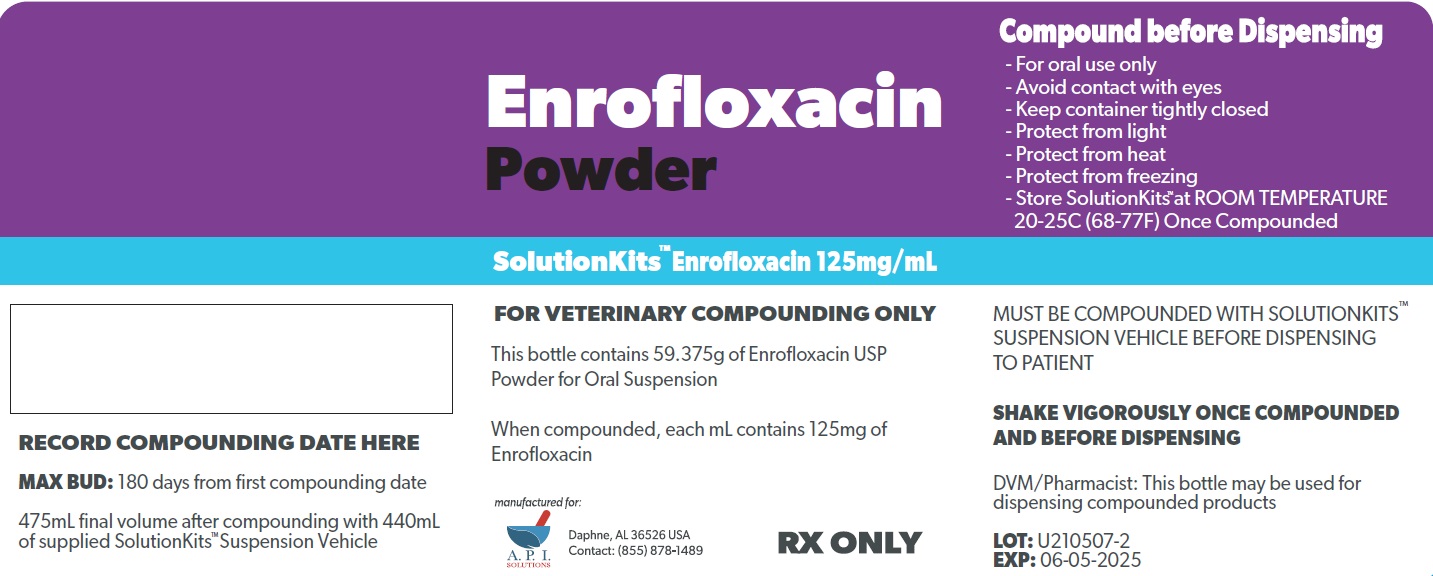
SolutionKits™Enrofloxacin
Enrofloxacin 125 mg/mL in SolutionKits™ Suspension Vehicle
FOR VETERINARY COMPOUNDING ONLY
Each kit includes:
1 bottle containing Enrofloxacin Powder equivalent to 59.375g Enrofloxacin USP for Oral Suspension
1 bottle containing 440 mL SolutionKits™ Suspension Vehicle provided as a suspending agent for compounding (Contains Artificial Sweet Flavoring)
This kit contains 1 funnel
A.P.I.
Solutions
Daphne, AL USA
16.1 FL OZ (475 mL) as dispensed
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SOLUTIONKITS ENROFLOXACIN
enrofloxacin kitProduct Information Product Type Item Code (Source) NDC:46144-605 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46144-605-01 1 in 1 BOX Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 59.375 g Part 2 1 BOTTLE, PLASTIC 440 mL Part 1 of 2 SOLUTIONKITS ENROFLOXACIN
enrofloxacin powder, for suspensionProduct Information Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENROFLOXACIN (UNII: 3DX3XEK1BN) (ENROFLOXACIN - UNII:3DX3XEK1BN) ENROFLOXACIN 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 59.375 g in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BULK INGREDIENT FOR ANIMAL DRUG COMPOUNDING Part 2 of 2 ORAL SUSPENSION VEHICLE
suspension liquidProduct Information Route of Administration NOT APPLICABLE Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) COCONUT OIL (UNII: Q9L0O73W7L) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) POLYGLYCERYL-3 OLEATE (UNII: XRQ165498B) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 440 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BULK INGREDIENT FOR ANIMAL DRUG COMPOUNDING Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BULK INGREDIENT FOR ANIMAL DRUG COMPOUNDING 01/03/2022 Labeler - API Solutions (831870824)