Label: MCKESSON PVP PREP SOLUTION- povidone-iodine liquid
-
NDC Code(s):
68599-3500-1,
68599-3500-2,
68599-3500-5,
68599-3500-6, view more68599-3502-1, 68599-3502-2, 68599-3502-5, 68599-3502-6
- Packager: McKesson Medical-Surgical
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not
- use in the eyes
- use as a first aid antiseptic Longer than one week
- use on individuals who are allergic or sensitive to iodine
- use on deep or puncture wounds
- use on animal bites
- use on serious burns
- apply over Large areas of the body
- Directions
- Other Information
- Inactive ingredient
- Principal Display Panel - McKesson PVP Prep Solution 4 fl oz Carton label
-
Principal Display Panel - McKesson PVP Prep Solution 4 fl oz label
NDC 68599-3502-2
McKESSON
PVP Prep Solution USP
10% POVIDONE IODINE
NET CONTENTS
4 fl oz (118 mL)
36 BOTTLES PER CASE
Do not store above 77°F (25°C).
Do not store below 68°F (20°C).
Not made with natural rubber latex.
Distributed By
McKesson Medical-Surgical Inc.
Richmond. VA 23233
PVN B0520 Made in Mexico
LOT
EXP
MFR# 039

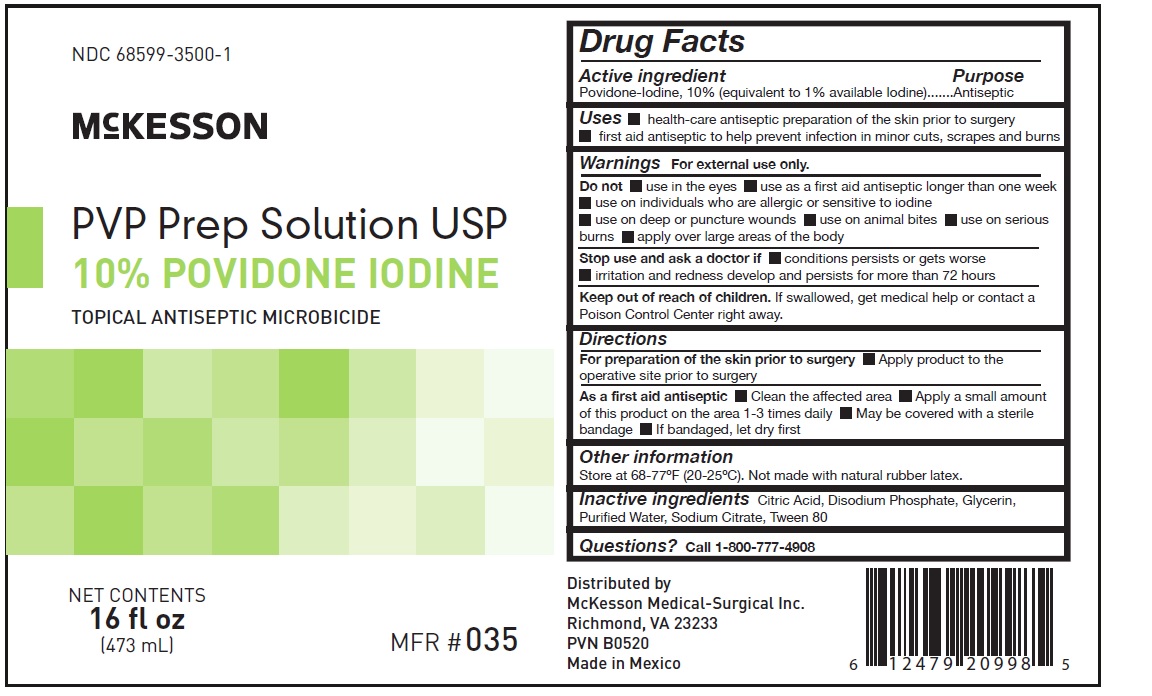
- Principal Display Panel - McKesson PVP Prep Solution 16 fl oz Carton label
-
Principal Display Panel - McKesson PVP Prep Solution 16 fl oz label
 NDC 68599-3500-2
NDC 68599-3500-2
McKESSON
PVP Prep Solution USP
10% POVIDONE IODINE
NET CONTENTS
16 fl oz (473 mL)
12 BOTTLES PER CASE
Do not store above 77°F (25°C).
Do not store below 68°F (20°C).
Not made with natural rubber latex.
Distributed By
McKesson Medical-Surgical Inc.
Richmond. VA 23233
PVN B0520 Made in Mexico
LOT
EXP
MFR# 035
- Principal Display Panel - McKesson PVP Prep Solution 1 gal Carton label
-
Principal Display Panel - McKesson PVP Prep Solution 1 gal label
NDC 68599-3500-6
McKESSON
PVP Prep Solution USP
10% POVIDONE IODINE
NET CONTENTS
1 gal (3.8 L)
4 BOTTLES PER CASE
Do not store above 77°F (25°C).
Do not store below 68°F (20°C).
Not made with natural rubber latex.
Distributed By
McKesson Medical-Surgical Inc.
Richmond. VA 23233
PNV B0520 Made in Mexico
LOT
EXP
MFR# 036

- Principal Display Panel - McKesson PVP Prep Solution 8 fl oz. label
-
Principal Display Panel - McKesson PVP Prep Solution 8 fl. oz. Carton Label
NDC 68599-3502-6
McKESSON
PVP Prep Solution USP
10% POVIDONE IODINE
NET CONTENTS
8 fl oz (237 mL)
24 BOTTLES PER CASE
Do not store above 77°F (25°C).
Do not store below 68°F (20°C).
Not made with natural rubber latex.
Distributed By
McKesson Medical-Surgical Inc.
Richmond. VA 23233
PVN B0520 Made in Mexico
LOT
EXP
MFR# 034

-
INGREDIENTS AND APPEARANCE
MCKESSON PVP PREP SOLUTION
povidone-iodine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68599-3502 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 1 mg in 10 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68599-3502-2 36 in 1 CASE 10/20/2016 1 NDC:68599-3502-1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68599-3502-6 24 in 1 CASE 03/13/2017 2 NDC:68599-3502-5 237 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 10/17/2016 MCKESSON PVP PREP SOLUTION
povidone-iodine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68599-3500 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 1 mg in 10 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68599-3500-2 12 in 1 CASE 10/20/2016 1 NDC:68599-3500-1 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68599-3500-6 4 in 1 CASE 10/20/2016 2 NDC:68599-3500-5 3800 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 10/17/2016 Labeler - McKesson Medical-Surgical (023904428) Establishment Name Address ID/FEI Business Operations Degassa 812771980 manufacture(68599-3502, 68599-3500)









 NDC 68599-3502-1
NDC 68599-3502-1