Label: TERUFLEX BLOOD BAG SYSTEM WITH DIVERSION BLOOD SAMPLING ARM ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD) AND OPTISOL (AS-5) RED CELL PRESERVATIVE (anticoagulant citrate phosphate dextrose- cpd and as-5 red cell preservative kit
- NDC Code(s): 53877-008-71
- Packager: Terumo Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 6, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
1. INDICATIONS AND USAGE
1.1. Read these instructions carefully before use.
1.2. Rx ONLY.
1.3. Intended for the collection, processing and preservation of Whole Blood and blood components. Not intended for direct intravenous infusion.
1.4. For the collection of 450 mL ±10% or 500 mL ±10% Whole Blood.
1.5. Integral Diversion Blood Sampling Arm is intended to divert and obtain donor samples for laboratory testing prior to collection of the Whole Blood unit.
1.6. For further processing, use standard component processing techniques.
-
2. DOSAGE AND ADMINISTRATION
2.1. To open blister package, peel cover film back 4/5 of its length.
2.2. Prepare the blood bag following your institution's standard operating procedures.
2.2.1. Materials Needed:
- VENOJECT®@Tube Holder (code P-1316R) or equivalent
- VENOJECT@Multi-Sample Luer Adapter (code MN*2000T) or equivalent
- Evacuated blood collection tubes (glass or plastic)
2.3. Make a loose knot in the donor tubing below the "Y" and CLIKTIP® (inline closure device) unless alternate methods are used to seal the tubing at the end of collection.
2.4. Temporarily clamp donor tubing between the phlebotomy needle and the "Y".
2.5. Close the White Clamp below the diversion pouch.
2.6. Assemble the luer adapter and the tube holder.
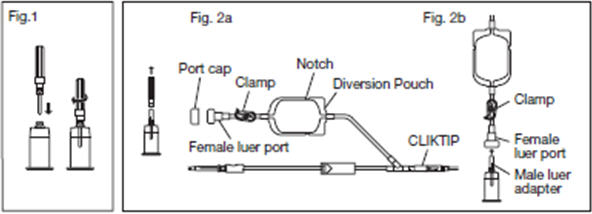
2.6.1. Connect the VENOJECT@Multi-Sample Luer Adapter to the VENOJECT@Tube Holder (or equivalent) (Fig. 1).
2.6.2. Twist and snap to remove the blue port cap at the end of the Diversion Blood Sampling Arm (Fig. 2a).
2.6.3. Insert the Holder/Luer assembly in the female luer port (Fig. 2b).
2.6.4. NOTE: Alternatively, steps 2.6.1., 2.6.2., and 2.6.3. (above) may be performed at any time during bag preparation or after the blood is collected into the diversion pouch.
2.7. Suspend the collection bag as far as possible below the donor's arm.
2.8. Apply blood pressure cuff or tourniquet to donor's arm. Disinfect site of phlebotomy. If blood pressure cuff is used, inflate to approximately 60 mmHg.
2.9. Remove the needle cover and perform phlebotomy. Remove the temporary clamp on the donor tubing to permit blood flow into the Diversion Blood Sampling Arm pouch.
2.9.1. CAUTION: Do not touch the needle after removing the needle cover.
2.9.2. CAUTION: Assure that the White Clamp below the pouch is closed prior to initiating phlebotomy.
2.10. Secure the needle guard device in place following the device instructions provided on the reverse side.
2.11. Secure donor tubing to donor's arm.
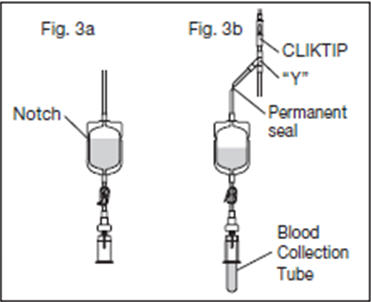
2.12. Position the diversion pouch with the notches up and the Tube Holder/Luer Adapter assembly (or port cap) down. When the level of blood in the pouch is approximately in line with the notches, the diversion pouch is full (Fig. 3a).
2.12.1. NOTE: The approximate fill volume of the pouch at the notches is 35 mL.
2.13. Permanently seal the tubing between the "Y" and the diversion pouch to maintain a closed system using an aluminum clip or a tube sealer approved for use with tubing connected to a donor (Fig. 3b).
2.13.1. CAUTION: Do not use a dielectric tube sealer to seal the tubing while the needle is connected to the donor's body unless it is approved for such a purpose.
2.14. To initiate blood flow into the collection bag, break the CLIKTIP between the "Y" and the collection bag.
2.15. To avoid clot formation, collect samples as soon as possible from the diversion pouch as follows (Fig. 3b).
2.15.1. CAUTION: Do not collect donor test samples until the tubing between the "Y" and the diversion pouch is permanently sealed.
2.15.2. Open the White Clamp on the tubing below the pouch to open the pathway for sampling.
2.15.3. Position the diversion pouch with the notches up and the Tube Holder/Luer Adapter assembly downward. Assure that any air in the pouch is at the top and will not enter the blood collection tubes.
2.15.4. Insert blood collection tube firmly into the tube holder; when full, remove sample tube from holder. Repeat to collect additional samples.
2.15.5. NOTE: The pouch may be removed after the donor test samples are collected.
A second seal must be made between the diversion pouch and the permanent seal prior to removing the pouch.
2.16. Mix blood with anticoagulant in the collection bag and continue to mix at several intervals during collection and immediately after collection. If using an automated mixer, follow manufacturer's instructions.
2.17. Collect labeled volume of blood 450 mL ±10% or 500 mL ±10%.
2.18. When the desired amount of blood has been collected, seal the tubing or tighten the loose knot (white knot) prepared in Step 2.3. Make a second seal between the first seal or knot and the "Y". Various methods may be used to seal tubing.
2.19. Release pressure on the donor's arm and remove the needle into the needle guard device following the device instructions provided on the reverse side. Sever the donor tubing between the two seals previously made below the CLIKTIP and "Y".
2.19.1. CAUTION: Discard the Diversion Blood Sampling Arm and phlebotomy needle/donor tubing according to institutional standard operating procedures.
2.20. Strip blood from donor tubing into collection bag, mix well, and allow tubing to refill; repeat once. To prevent the blood from clotting in the tubing, work quickly as possible. Make an appropriate number of segments of anticoagulated blood for testing by sealing on or near the X marks. Leave segments attached to the Whole Blood unit.
2.21. The time between Whole Blood collection and component separation may vary depending on both the blood bag system and processing options selected. Follow your institution's standard operating procedures to prepare components.
2.21.1. If the Whole Blood is to be processed into room temperature components, maintain the blood at ambient temperature.
2.21.2. If the Whole Blood is to be processed into other components (including Plasma Frozen Within 24 Hours After Phlebotomy), Whole Blood must either be placed in storage at a temperature between 1-6°C within 8 hours of blood collection or cooled towards a temperature between 1-10°C (e.g. during transport) and then placed in storage at a temperature between 1-6°C upon arrival at the processing center.
2.22. Platelet Rich Plasma should be separated from the Red Blood Cells within 8 hours of blood collection, if prepared.
2.23. Plasma intended for production of Fresh Frozen Plasma should be separated from the Red Blood Cells and placed in a freezer at –18°C or colder within 8 hours of blood collection.
2.24. Plasma intended for production of Plasma Frozen Within 24 Hours After Phlebotomy (PF24) should be placed in a freezer at –18°C or colder within 24 hours of blood collection.
2.25. OPTISOL should be added to the Red Blood Cells immediately after removal of the plasma. If plasma is not separated from the Red Blood Cells within 8 hours, OPTISOL may be added within 72 hours of collection if Whole Blood is refrigerated.
2.26. For further preparation and processing of other plasma components, use standard processing and storage techniques following approved regulations and standards.
2.27. Select the appropriate spin condition and centrifuge Whole Blood unit to separate CPD Red Blood Cells from plasma or platelet rich plasma, as appropriate.
2.28. Break the CLIKTIP of primary collection bag and transfer plasma into satellite bag, or transfer platelet rich plasma into XT-612® Platelet bag. Clamp transfer tubing of satellite bag.
2.29. Break the CLIKTIP of the OPTISOL Solution bag and drain the contents into the primary collection bag containing Red Blood Cells.
2.29.1. NOTE: For TERUFLEX double blood bag sets, seal tubing of the OPTISOL bag in two places, cut between seals and separate from satellite bag(s). Discard OPTISOL Solution bag.
2.29.2. NOTE: For TERUFLEX triple and quadruple blood bag sets, the empty OPTISOL bag may now be used for further component preparation.
2.30. Seal tubing of primary collection bag in two places, cut between seal, and if applicable, separate from satellite bag(s).
2.31. Invert the Red Blood Cell -OPTISOL mixture several times to assure the final product is well suspended.
2.32. Store AS-5 Red Blood Cells between 1–6°C for up to 42 days.
2.32.1. NOTE: Whole Blood or Red Blood Cells in CPD may be stored for up to 21 days at 1-6°C.
2.33. Store Platelets, Leukocytes Reduced between 20-24°C, maintaining a continuous gentle agitation, for up to 5 days in XT-612 bag.
-
3. DOSAGE FORMS AND STRENGTHS
3.1. 63 mL Citrate Phosphate Dextrose (CPD) anticoagulant USP for collection of 450 mL Whole Blood. Each 63 mL contains 1.61 g Dextrose (monohydrate) USP, 1.66 g Sodium Citrate (dihydrate) USP, 188 mg Citric Acid (anhydrous) USP, 140 mg Monobasic Sodium Phosphate (monohydrate) USP.
3.2. 70 mL Citrate Phosphate Dextrose (CPD) anticoagulant USP for collection of 500 mL Whole Blood. Each 70 mL contains 1.79 g Dextrose (monohydrate) USP, 1.84 g Sodium Citrate (dihydrate) USP, 209 mg Citric Acid (anhydrous) USP, 156 mg Monobasic Sodium Phosphate (monohydrate) USP.
3.3. 100 mL OPTISOL Red Cell Preservative Solution. Each 100 mL contains 877 mg Sodium Chloride USP, 900 mg Dextrose (monohydrate) USP, 525 mg Mannitol USP, 30 mg Adenine USP.
3.4. 111 mL OPTISOL Red Cell Preservative Solution. Each 111 mL contains 974 mg Sodium Chloride USP, 1.00g Dextrose (monohydrate) USP, 583 mg Mannitol USP, 33.3 mg Adenine USP.
-
5. WARNINGS AND PRECAUTIONS
5.1. Rx ONLY.
5.2. Do not use unless solutions are clear and free from particulates.
5.3. Always inspect the blood bag set for leaks before use.
5.4. Avoid excessive heat and direct sunlight. Protect from freezing.
5.5. Recommended storage conditions: Room Temperature (15-30°C/59-86°F).
5.6. It is normal to have condensation in the blister packaging. If the amount of moisture is greater than expected, check for leaks from the fluid-filled components of the blood bag set.
5.7. Use aseptic techniques.
5.8. Do not use a dielectric tube sealer to seal the tubing while the needle is connected to the donor's body unless it is approved for such a purpose.
5.9. Do not touch needle after removing the needle cover.
5.10. Assure that the White Clamp below the pouch is closed prior to initiating phlebotomy.
5.11. Do not collect donor test samples until the tubing between the "Y" and the diversion pouch is permanently sealed.
5.12. Discard the Diversion Blood Sampling Arm and phlebotomy needle/donor tubing according to institutional standard operating procedures.
5.13. The AGELESS® oxygen absorber packet, (Mitsubishi Gas Chemical) contained in this package absorbs oxygen and generates heat on removal. Do not open and handle it with care.
5.14. Dispose of the AGELESS packet with the blister tray.
5.15. Do not dispose the AGELESS packet with wastes containing volatile or flammable materials.
5.16. Due to possible exposure to infectious agents in the handling of blood, take adequate precautions at all times to prevent exposure to and transmission of such agents. Follow your institution's standard operating procedures.
-
11. DESCRIPTION / PRODUCT SPECIFICATIONS
11.1. This blood bag system includes a 16 gauge × 1 1/2 inch (1.60 × 38 mm) needle with needle cover and either a 450mL or 500mL (nominal capacity 600mL) primary collection bag containing 63 mL or 70mL, respectively, Citrate Phosphate Dextrose (CPD) anticoagulant. The Double blood bag set has one integrally attached empty satellite bag and one satellite bag containing 100 mL or 111 mL, respectively, OPTISOL Red Cell Preservative Solution. The Triple blood bag set has one empty XT-612 5 day Platelet bag and one satellite bag containing 100 mL or 111 mL, respectively, OPTISOL Red Cell Preservative Solution. The Quadruple blood bag set has one integrally attached empty XT-612 5 day Platelet bag, one empty satellite bag, and one satellite bag containing 100 mL or 111 mL, respectively, OPTISOL Red Cell Preservative Solution.
11.2. Blood bag codes ending in A2 are supplied with Integral Diversion Blood Sampling Arm intended to divert and obtain donor samples for laboratory testing prior to collection of the Whole Blood unit.
11.3. Blood bag codes ending in A2 also include a DonorCare® Needle Guard pre-attached to the donor tubing. DonorCare Needle Guard device instructions are provided on the reverse side.
11.4. The blood bag collection set is made of PVC (polyvinyl chloride with DEHP plasticizer).
11.5. The blood bag has no components made of natural rubber latex.
11.6. Tubing internal diameter (ID) nominal 3.0 mm.
11.7. Tubing outer diameter (OD) nominal 4.4 mm.
11.8. Donor tubing line maximum 16 segments available.
-
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. Single use only.
16.2. Sterile and non-pyrogenic fluid path. Sterilized by steam. Opacity of the blood bag system may be observed. This is due to moisture absorption during the sterilization process. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
16.3. A Material Safety Data Sheet (MSDS) is not required for this product.
16.4. Recommended storage conditions: Room Temperature (15-30°C/59-86°F).
16.5. Avoid excessive heat and direct sunlight. Protect from freezing.
16.6. To open blister package, peel cover film back 4/5 of its length.
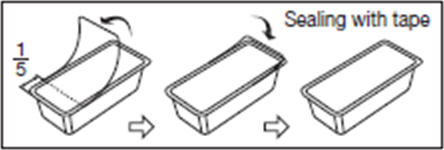
16.7. After opening the blister package, unused blood bags may be stored at room temperature for 96 hours or they may be stored for 30 days by returning cover film to original position and sealing with tape to prevent evaporation of solutions.
16.8. Blood bags in the unopened blister package may be used through the last day of the month and year as indicated on the original manufacturer's packaging.
16.9. The AGELESS packet contained in this package absorbs oxygen and generates heat on removal. Do not open and handle it with care.
16.10. Dispose of the AGELESS packet with the blister tray.
16.11. Do not dispose the AGELESS packet with wastes containing volatile or flammable materials.
16.12. For the Double blood bag sets, Codes BB*AGD456A2 and BB*AGD506A2 are supplied 24/case.
16.13. For the Triple blood bag sets, Codes BB*AGT456A2 and BB*AGT506A2 are supplied 24/case.
16.14. For the Quadruple blood bag sets, Codes BB*AGQ456A2 and BB*AGQ506A2 are supplied 18/case.
-
SPL UNCLASSIFIED SECTION
MANUFACTURED BY:
TERUMO CORPORATION
44-1, 2-CHOME, HATAGAYA, SHIBUYA-KU,
TOKYO 151-0072, JAPAN MADE IN JAPAN
(R): Registered Trademark
©TERUMO CORPORATION December, 2015
DonorCare is a registered trademark of Noble House Group Pty. Ltd. CORPORATION.
AGELESS is a registered trademark of MITSUBISHI GAS CHEMICAL CO., INC.
-
PROCEDURE FOR USE OF DonorCare® Needle Guard
This device is for use by trained individuals.
Intended Use
The DonorCare® Needle Guard is incorporated onto the donor tubing to shield the needle immediately after withdrawal from the donor.
Single Use Only.
Preparation
1. Move the DonorCare on the tubing ensuring that it slides easily and the arrow is pointing toward the needle hub.
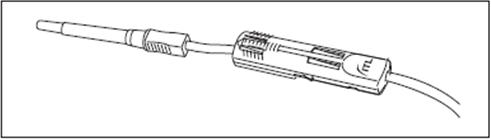
2. Ensure that the three lock points on the DonorCare are locked closed.
Whole Blood Collection
3. Perform the phlebotomy as per your institution's standard operating procedures.
4. Slide the DonorCare over the needle hub so that it covers approximately one half to two thirds of the needle hub.
5. Stabilize the DonorCare to the arm by placing a piece of tape over the front end so that the tape does not extend over the front of the DonorCare.
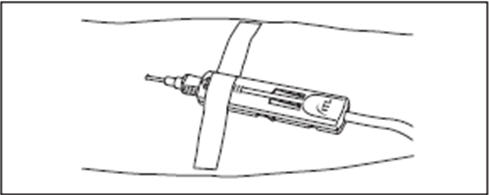
Withdrawal of Needle
Important
The DonorCare must be held stationary while the needle is withdrawn into it.
Caution
The needle must be fully shielded by DonorCare to prevent accidental injury.
6. Hold gauze over the venipuncture site with finger tips without exerting pressure. Hold the sides of the DonorCare near the front with the index finger and thumb of the same hand.
7. With the other hand, hold the donor tubing close behind the DonorCare.
Note: A hemostat may be placed on the tubing behind the DonorCare when the blood collection is complete.
This will help to prevent blood drops from forming.
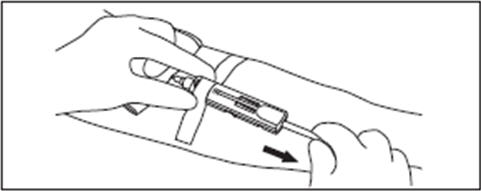
8. Pull tubing smoothly and swiftly with one motion until the needle is locked in place inside the DonorCare.
9. Confirm that the needle is locked by:
a. Listening for two 'clicks' as the needle is drawn into DonorCare.
b. If the clicks are not heard as the needle is drawn into the DonorCare, continue to pull firmly on the tubing to assure needle is fully withdrawn into DonorCare.
10. Visually check that the needle is fully shielded by DonorCare before removing from the donor's arm.
11. Remove the tape from the DonorCare and arm.
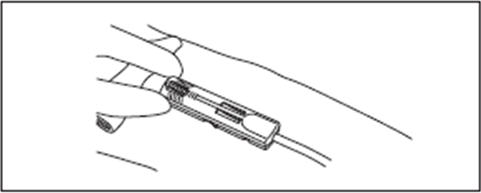
12. Apply pressure to the gauze covering the venipuncture site.
Warning
Do not place fingers at the opening of DonorCare after removal from the donor's arm.
Difficult Phlebotomy (Examples may include: Slow blood flow, deep or fine vein, steep angle) It may be necessary to delay placing the DonorCare over the needle hub until the end of the blood collection. In such situations:
Leave the DonorCare on the tubing behind the needle hub. At the end of the blood collection carefully remove the tape from the needle hub and slide the DonorCare over the hub so that it covers approximately one half to two thirds of the needle hub. Withdraw the needle into the DonorCare as stated in steps 6. through 12. above.
Rx ONLY
DonorCare is manufactured by ITL Corporation, Melbourne, Australia.
TERUMO CORPORATION
44-1, 2-CHOME, HATAGAYA, SHIBUYA-KU,
TOKYO 151-0072, JAPAN
© TERUMO CORPORATION December, 2015
-
PRINCIPAL DISPLAY PANEL
Tray/Case Label
TERUFLEX® BLOOD BAG SYSTEM with
DIVERSION BLOOD SAMPLING ARM®
CPD WITH OPTISOL® RED CELL PRESERVATIVE SOLUTION
FOR COLLECTION OF 500mL OF BLOOD
Each unit consists of a collection bag containing 70mL of Anticoagulant
CPD solution, with a satellite bag containing 111mL of OPTlSOL Red
Cell Preservative Solution.
Each 70mL Anticoagulant CPD solution USP contains 1.79g Dextrose
(monohydrate) USP, 1.84g Sodium Citrate (dihydrate) USP, 209mg Citric
Acid (anhydrous) USP, 156mg Monobasic Sodium Phosphate
(monohydrate) USP.
Each 111mL OPTISOL Red Cell Preservative Solution contains 974mg
Sodium Chloride USP, 1.00g Dextrose (monohydrate) USP, 583mg
Mannitol USP, 33.3mg Adenine USP.
STERILE, NON-PYROGENIC FLUID PATH.
DO NOT USE UNLESS ANTICOAGULANT IS CLEAR.
CODE
LOT No.
EXPIRY
UNITS
DONOR NEEDLE 16G x 1 1/2˝ (1.60 x 38mm)
Rx ONLY
RECOMMENDED STORAGE: Room Temperature (15-30°C/59-86°F).
Avoid excessive heat. Protect from freezing.
After opening, unused bags may be stored for 30 days by returning cover film to original
position and sealing with tape to prevent possible loss of moisture.
See Instructions For Blood Collection.
Manufactured by : TERUMO CORPORATION Tokyo, Japan
® : Registered Trademark
Diversion Blood Sampling Arm is a trademark of TERUMO CORPORATION.
Issued 05/03
B-4-H6-A2 1
Place Label here
-
INGREDIENTS AND APPEARANCE
TERUFLEX BLOOD BAG SYSTEM WITH DIVERSION BLOOD SAMPLING ARM ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD) AND OPTISOL (AS-5) RED CELL PRESERVATIVE
anticoagulant citrate phosphate dextrose (cpd) and as-5 red cell preservative kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53877-008 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53877-008-71 18 in 1 CASE 1 1 in 1 BAG Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BAG 70 mL Part 2 1 BAG 111 mL Part 1 of 2 ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD)
anticoagulant citrate phosphate dextrose (cpd) solutionProduct Information Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Trisodium Citrate Dihydrate (UNII: B22547B95K) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 26.3 g in 1000 mL Sodium Phosphate, Monobasic (UNII: 3980JIH2SW) (Phosphate Ion - UNII:NK08V8K8HR, Sodium Cation - UNII:LYR4M0NH37) Sodium Phosphate, Monobasic 2.22 g in 1000 mL Dextrose Monohydrate (UNII: LX22YL083G) (Anhydrous Dextrose - UNII:5SL0G7R0OK) Dextrose Monohydrate 25.5 g in 1000 mL Anhydrous Citric Acid (UNII: XF417D3PSL) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 2.99 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 70 mL in 1 BAG; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN880217 05/05/2010 Part 2 of 2 OPTISOL RED CELL PRESERVATIVE
as-5 red cell preservative solutionProduct Information Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Chloride (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) Sodium Chloride 877 mg in 100 mL Dextrose Monohydrate (UNII: LX22YL083G) (Anhydrous Dextrose - UNII:5SL0G7R0OK) Dextrose Monohydrate 900 mg in 100 mL Mannitol (UNII: 3OWL53L36A) (Mannitol - UNII:3OWL53L36A) Mannitol 525 mg in 100 mL Adenine (UNII: JAC85A2161) (Adenine - UNII:JAC85A2161) Adenine 30 mg in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 111 mL in 1 BAG; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN880217 05/05/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN880217 05/05/2010 Labeler - Terumo Corporation (690543319) Establishment Name Address ID/FEI Business Operations Terumo Corp. - Fujinomiya Factory 695214015 MANUFACTURE(53877-008) , STERILIZE(53877-008) , ANALYSIS(53877-008) , LABEL(53877-008)

