Label: SECURA MOISTURIZING CLEANSER- benzethonium chloride spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 69740-308-00, 69740-309-00 - Packager: Smith & Nephew Medical Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 23, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- ACTIVE INGREDIENT
- PURPOSE
- USES
- WARNINGS
- DIRECTIONS
- INACTIVE INGREDIENTS
- QUESTION OR COMMENTS?
-
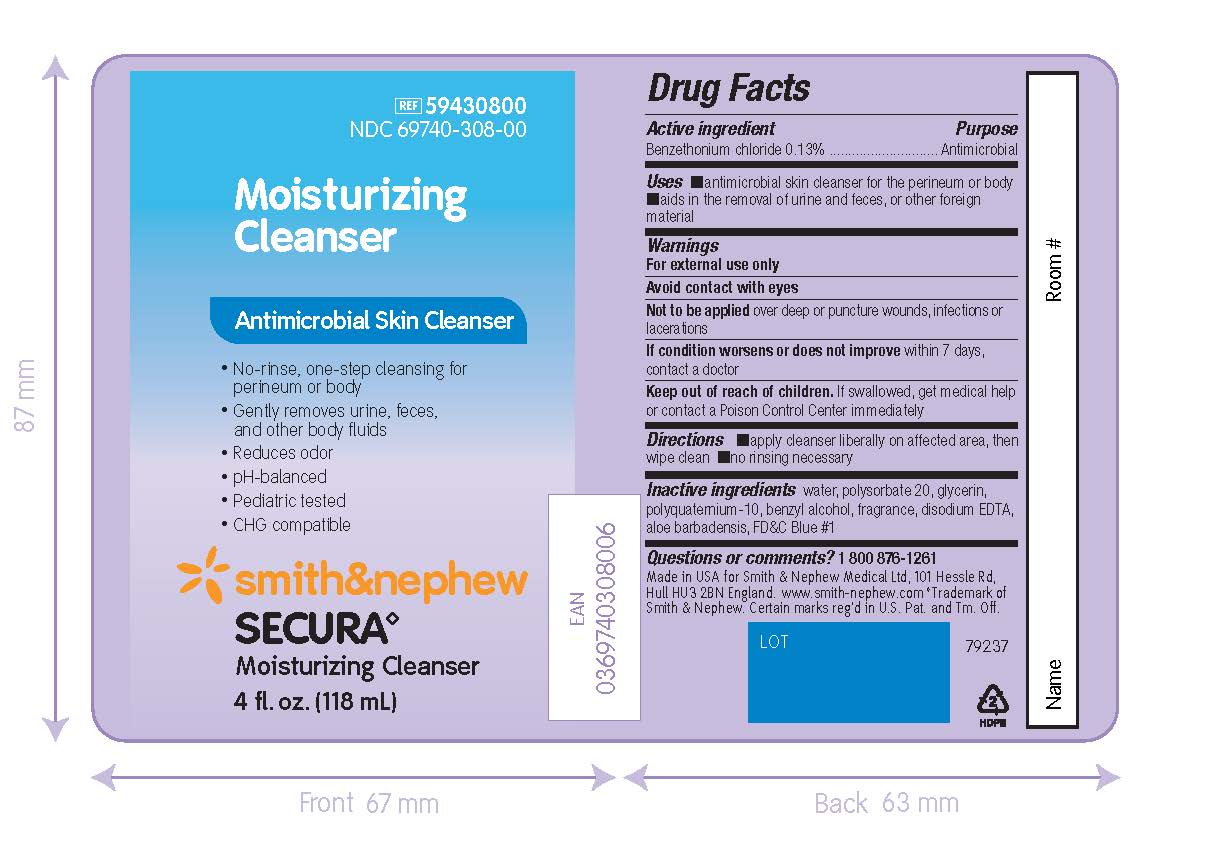
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - SECURA MOISTURIZING CLEANSER (118mL)
REF 59430800
NDC 69740-308-00
Moisturizing
CleanserAntimicrobial Skin Cleanser
- No-rinse, one-step cleansing for perineum or body
- Gently removes urine, feces, and other body fluids
- Reduces odor
- pH-balanced
- Pediatric-tested
- CHG compatible
Smith&Nephew
Secura◊
Moisturizing Cleanser4 fl. oz. (118 mL)
Made in the USA for
Smith & Nephew Medical Ltd,
101 Hessle Road
Hull, HU3 2BN, England
www.smith-nephew.com
◊Trademark of Smith & Nephew.Certain marks reg’d in US Patent Office and Tm. Off.
-
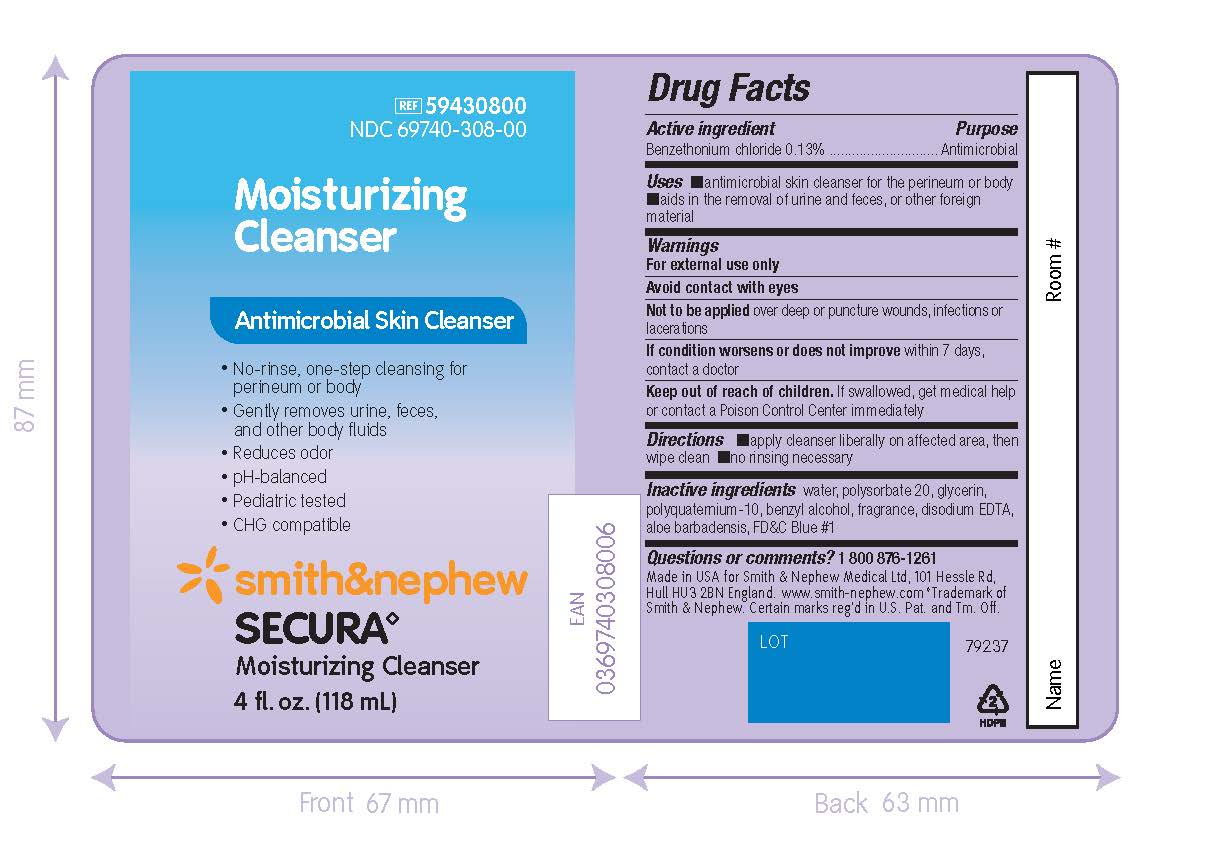
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - SECURA MOISTURIZING CLEANSER (236mL)
REF 59430900
NDC 69740-309-00
Moisturizing
CleanserAntimicrobial Skin Cleanser
- No-rinse, one-step cleansing for perineum or body
- Gently removes urine, feces, and other body fluids
- Reduces odor
- pH-balanced
- Pediatric-tested
- CHG compatible
Smith&Nephew
Secura◊
Moisturizing Cleanser8 fl. oz. (236 mL)
Made in the USA for
Smith & Nephew Medical Ltd,
101 Hessle Road
Hull, HU3 2BN, England
www.smith-nephew.com
◊Trademark of Smith & Nephew.Certain marks reg’d in US Patent Office and Tm. Off.

-
INGREDIENTS AND APPEARANCE
SECURA MOISTURIZING CLEANSER
benzethonium chloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69740-309 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 1.3 ug in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 2 uL in 1 mL FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 0.001 uL in 1 mL ALOE (UNII: V5VD430YW9) 0.05 uL in 1 mL GLYCERIN (UNII: PDC6A3C0OX) 10 uL in 1 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 30 uL in 1 mL WATER (UNII: 059QF0KO0R) 945.94 uL in 1 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 0.2 uL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69740-309-00 236 mL in 1.0 BOTTLE, SPRAY; Type 0: Not a Combination Product 08/01/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/01/2003 SECURA MOISTURIZING CLEANSER
benzethonium chloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69740-308 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 1.3 ug in 1 mL Inactive Ingredients Ingredient Name Strength ALOE (UNII: V5VD430YW9) 0.05 uL in 1 mL BENZYL ALCOHOL (UNII: LKG8494WBH) 2 uL in 1 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 0.2 uL in 1 mL FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 0.001 uL in 1 mL GLYCERIN (UNII: PDC6A3C0OX) 10 uL in 1 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 30 uL in 1 mL WATER (UNII: 059QF0KO0R) 945.94 uL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69740-308-00 118 mL in 1.0 BOTTLE, SPRAY; Type 0: Not a Combination Product 08/01/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/01/2003 Labeler - Smith & Nephew Medical Ltd (216344051) Establishment Name Address ID/FEI Business Operations Swiss American CDMO LLC 080170933 MANUFACTURE(69740-309, 69740-308)