Label: LORATADINE 10MG tablet
- NDC Code(s): 25000-092-03, 25000-092-79, 25000-092-80, 25000-092-81
- Packager: MARKSANS PHARMA LIMITED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
- WARNINGS
- DO NOT USE
- ASK A DOCTOR BEFORE USE IF
- WHEN USING THIS PRODUCT
- STOP USE AND ASK DOCTOR IF
- PREGNANCY/BREASTFEEDING
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
-
OTHER REQUIRED WARNINGS
Other information
- For Bottle pack: Tamper-evident: do not use this product if the imprinted foil seal over the mouth of the bottle is cut, torn, broken or missing.
- For Blister pack: Tamper-evident: do not use if the individual blister unit is open or torn
- store between 20º to 25º C (68º to 77º F)
- FDA approved dissolution test specifications differ from USP
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
NDC: 25000-092-81
Loratadine Tablets USP, 10 mg
14's count Carton

NDC: 25000-092-03
Loratadine Tablets USP, 10 mg
30's count Carton
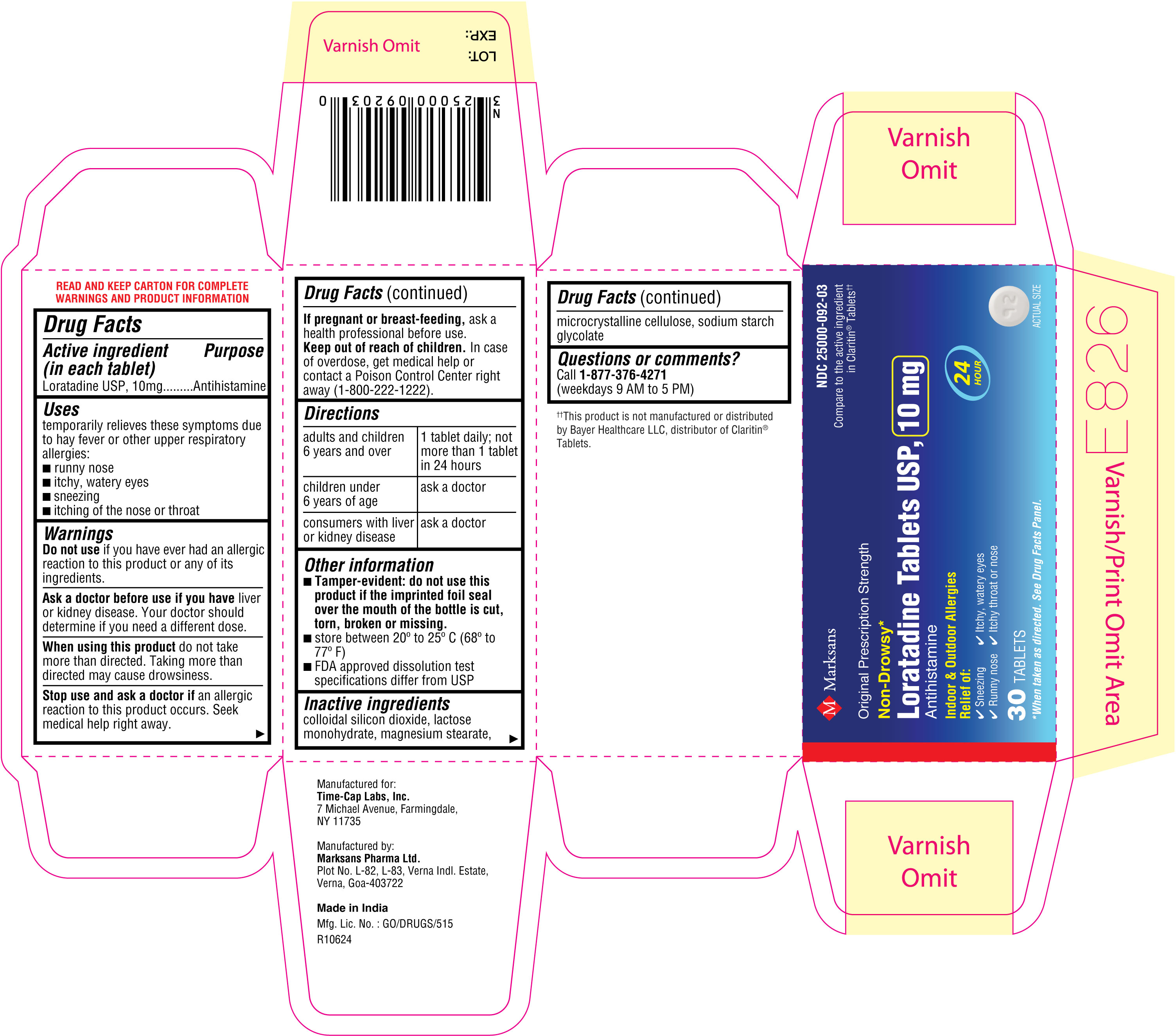
NDC: 25000-092-03
Loratadine Tablets USP, 10 mg
30's count Label

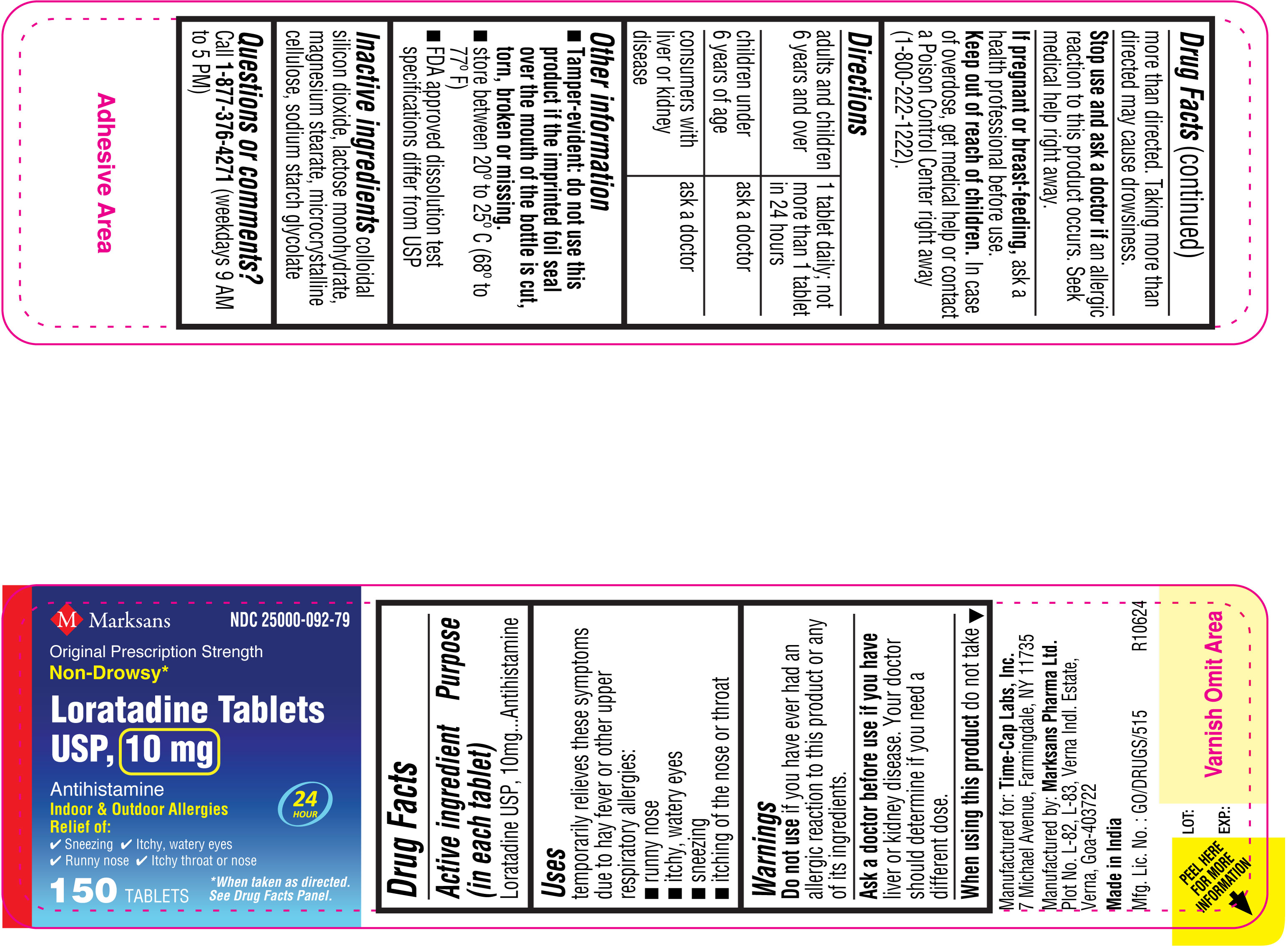
NDC: 25000-092-79
Loratadine Tablets USP, 10 mg
150's count Carton
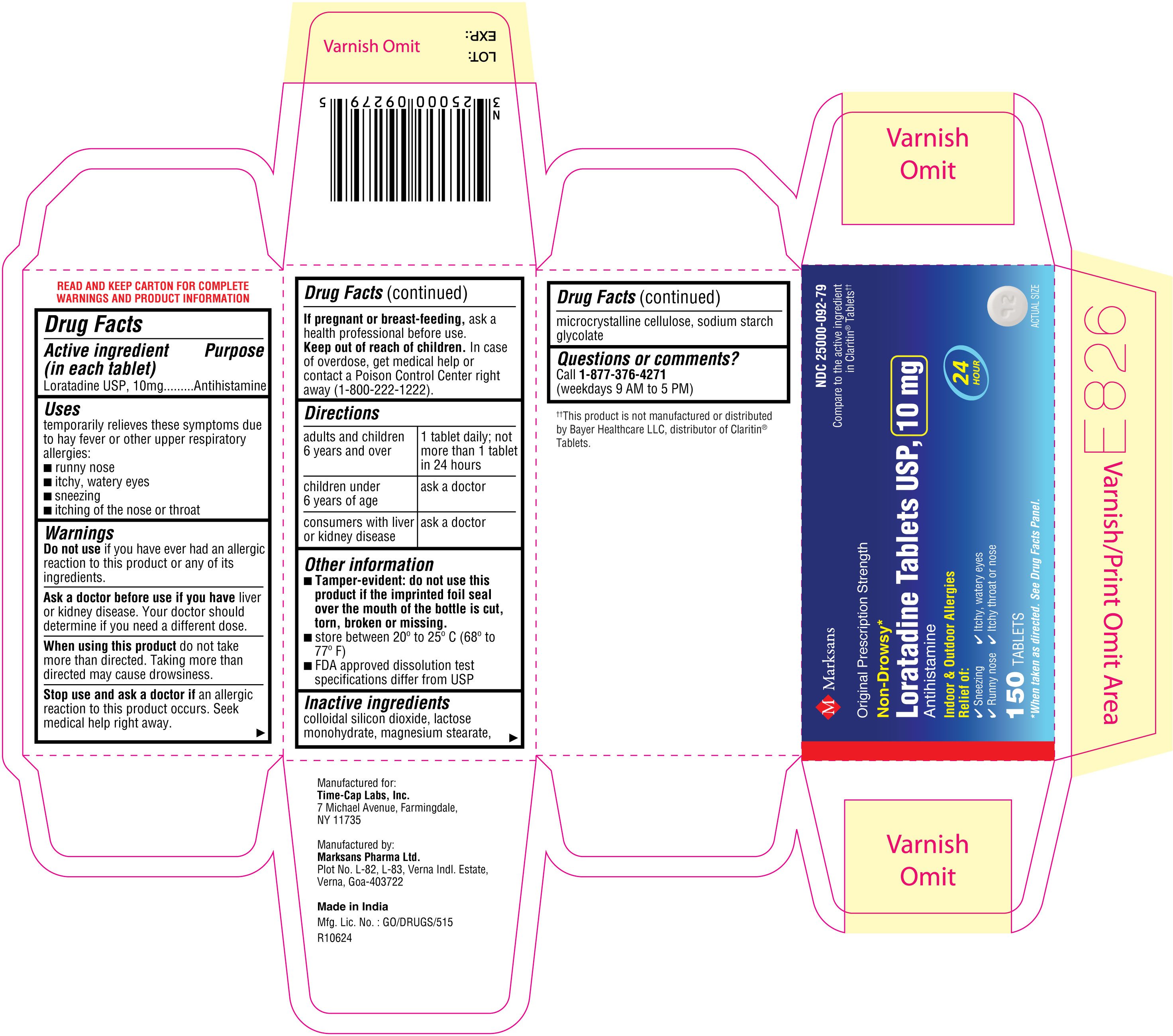
NDC: 25000-092-79
Loratadine Tablets USP, 10 mg
150's count Label
NDC: 25000-092-80
Loratadine Tablets USP, 10 mg
365's count Label
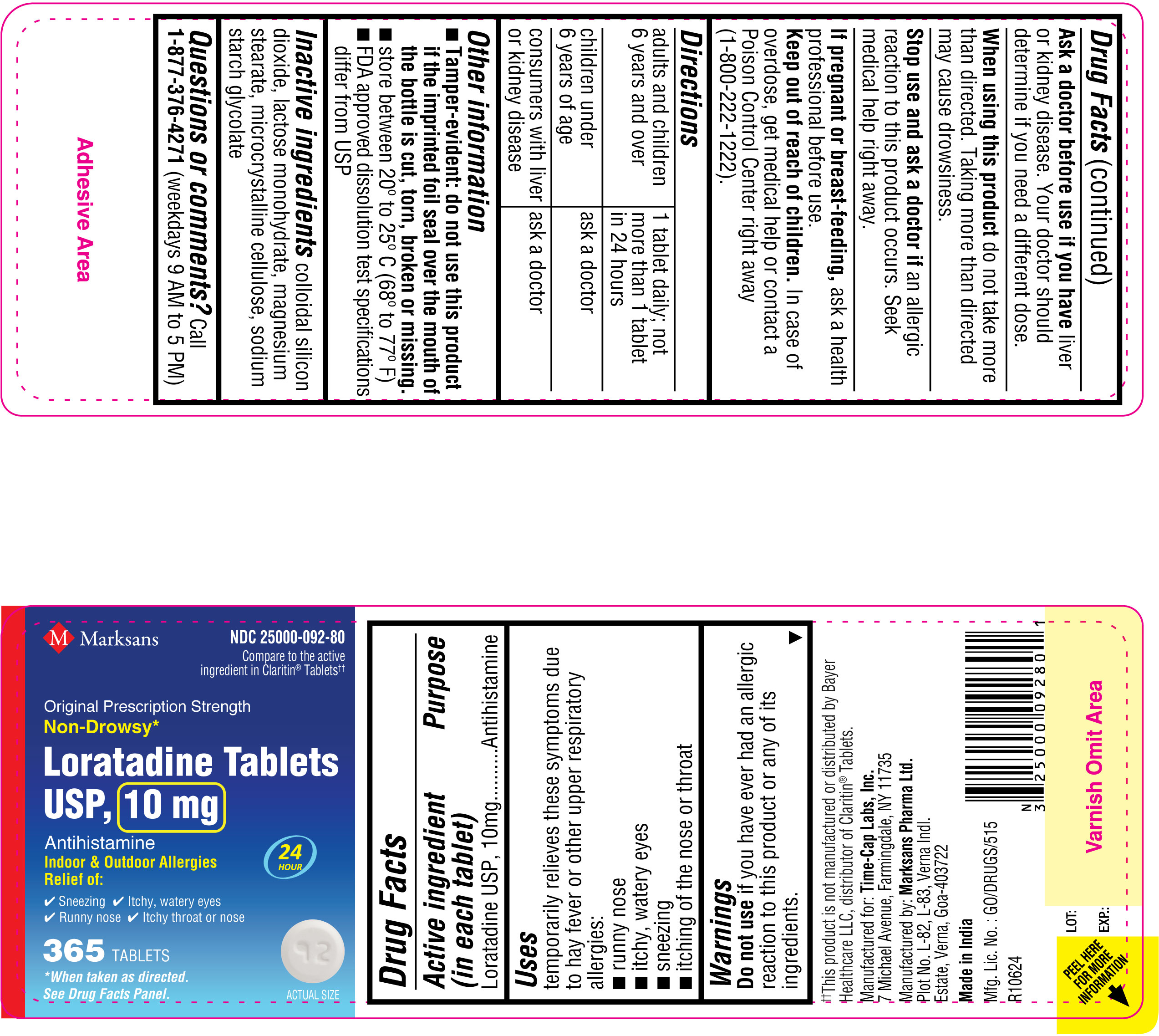
-
INGREDIENTS AND APPEARANCE
LORATADINE 10MG
loratadine 10mg tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:25000-092 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) Product Characteristics Color WHITE (White to off-white) Score no score Shape ROUND Size 6mm Flavor Imprint Code 92 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25000-092-03 1 in 1 CARTON 11/21/2024 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:25000-092-79 1 in 1 CARTON 11/21/2024 2 150 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:25000-092-81 2 in 1 CARTON 11/21/2024 3 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:25000-092-80 365 in 1 BOTTLE; Type 0: Not a Combination Product 11/21/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA219223 11/21/2024 Labeler - MARKSANS PHARMA LIMITED (925822975) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 925822975 MANUFACTURE(25000-092)






