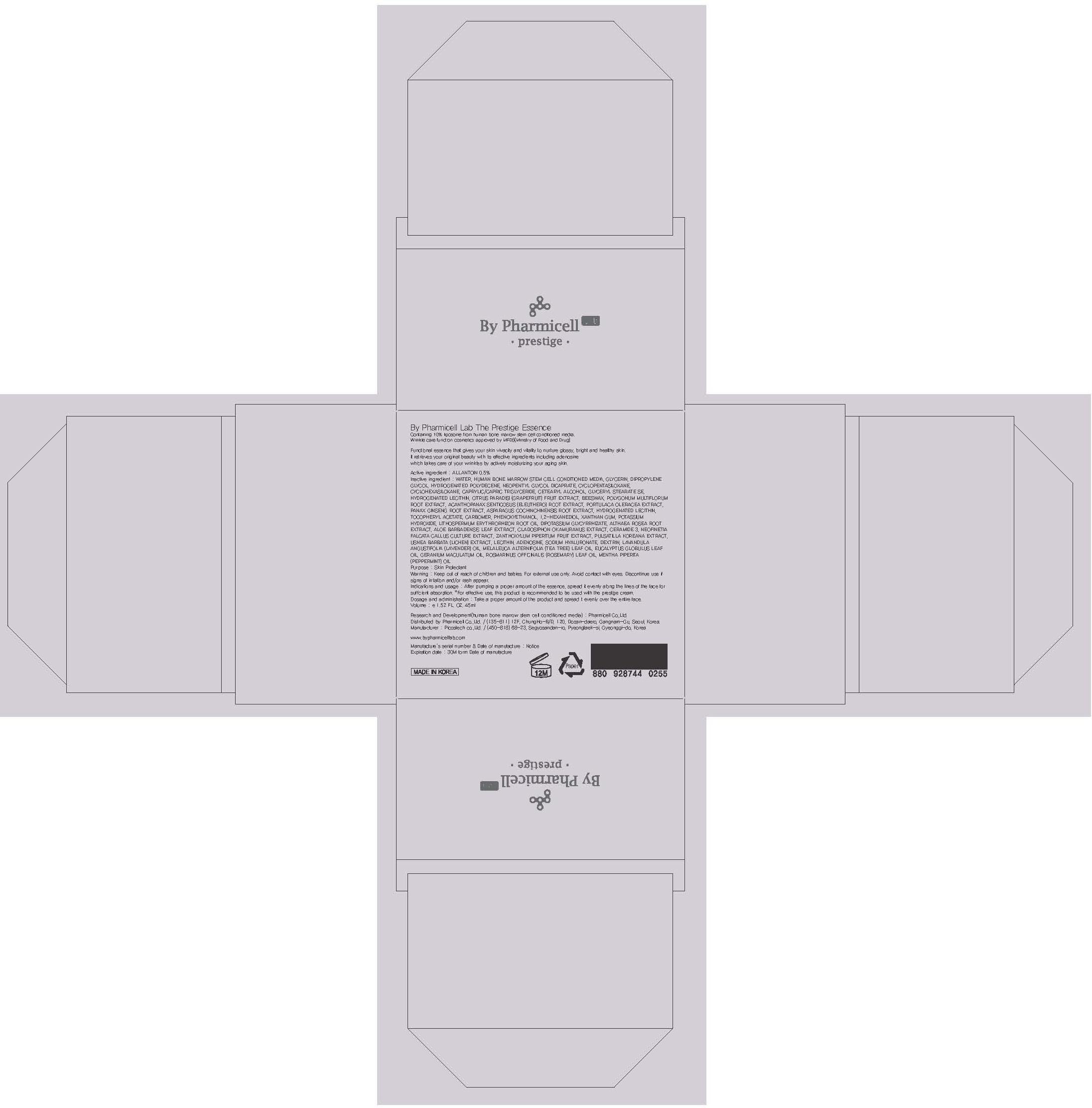
Label: BY PHARMICELL LAB THE PRESTIGE ESSENCE- allantoin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 60949-020-01 - Packager: Pharmicell Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 19, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive ingredient : WATER, HUMAN BONE MARROW STEM CELL CONDITIONED MEDIA, GLYCERIN, DIPROPYLENE GLYCOL, HYDROGENATED POLYDECENE, NEOPENTYL GLYCOL DICAPRATE, CYCLOPENTASILOXANE, CYCLOHEXASILOXANE, CAPRYLIC/CAPRIC TRIGLYCERIDE, CETEARYL ALCOHOL, GLYCERYL STEARATE SE, HYDROGENATED LECITHIN, CITRUS PARADISI (GRAPEFRUIT) FRUIT EXTRACT, BEESWAX, POLYGONUM MULTIFLORUM ROOT EXTRACT, ACANTHOPANAX SENTICOSUS (ELEUTHERO) ROOT EXTRACT, PORTULACA OLERACEA EXTRACT, PANAX GINSENG ROOT EXTRACT, ASPARAGUS COCHINCHINENSIS ROOT EXTRACT, HYDROGENATED LECITHIN, TOCOPHERYL ACETATE, CARBOMER, PHENOXYETHANOL, 1,2-HEXANEDIOL, XANTHAN GUM, POTASSIUM HYDROXIDE, LITHOSPERMUM ERYTHRORHIZON ROOT OIL, DIPOTASSIUM GLYCYRRHIZATE, ALTHAEA ROSEA ROOT EXTRACT, ALOE BARBADENSIS LEAF EXTRACT, CLADOSIPHON OKAMURANUS EXTRACT, CERAMIDE 3, NEOFINETIA FALCATA CALLUS CULTURE EXTRACT, ZANTHOXYLUM PIPERITUM FRUIT EXTRACT, PULSATILLA KOREANA EXTRACT, USNEA BARBATA (LICHEN) EXTRACT, LECITHIN, ADENOSINE, SODIUM HYALURONATE, DEXTRIN, LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL, MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL, EUCALYPTUS GLOBULUS LEAF OIL, GERANIUM MACULATUM OIL, ROSMARINUS OFFICINALIS (ROSEMARY) LEAF OIL, MENTHA PIPERITA (PEPPERMINT) OIL
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS AND USAGE
- DOSAGE AND ADMINISTRATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BY PHARMICELL LAB THE PRESTIGE ESSENCE
allantoin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60949-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 0.22 mg in 45 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60949-020-01 45 mL in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 10/01/2013 Labeler - Pharmicell Co., Ltd. (687744110) Registrant - Pharmicell Co., Ltd. (687744110) Establishment Name Address ID/FEI Business Operations Pharmicell Co., Ltd. 687744110 manufacture(60949-020)