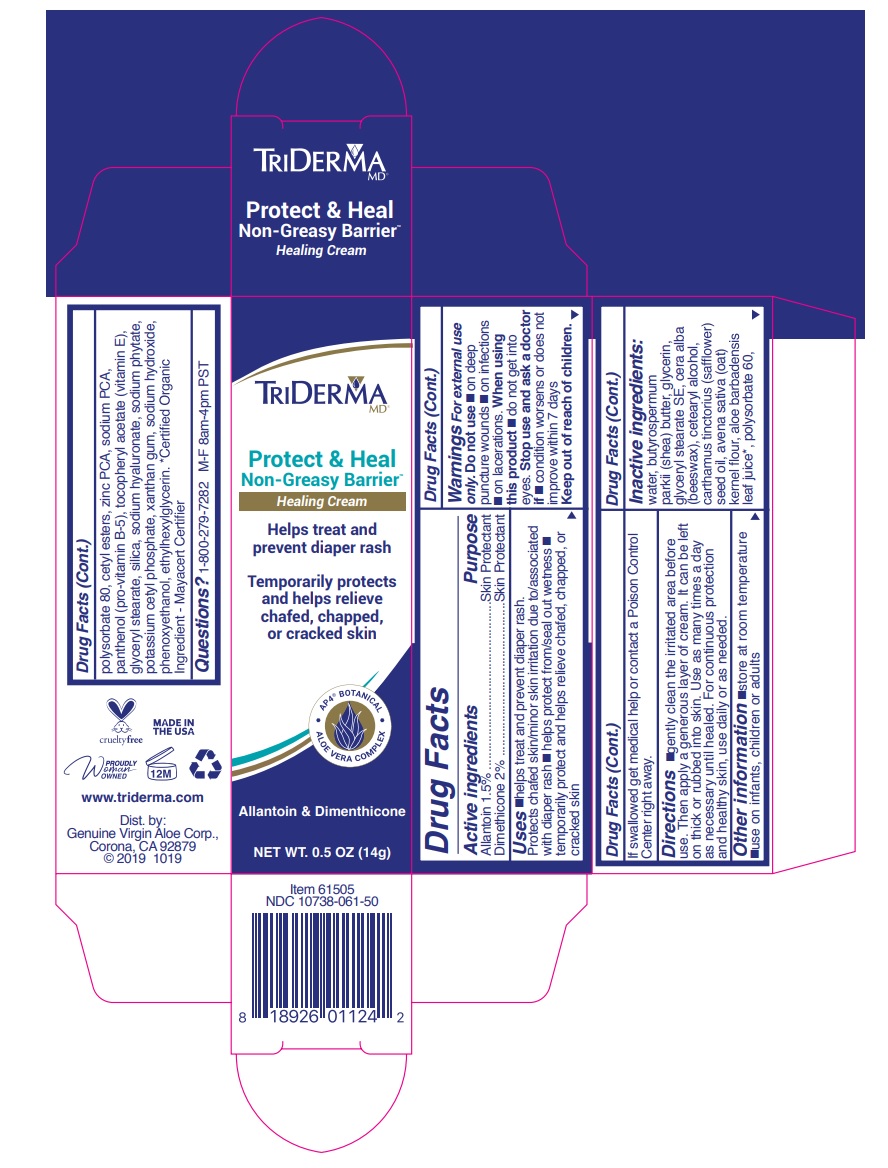
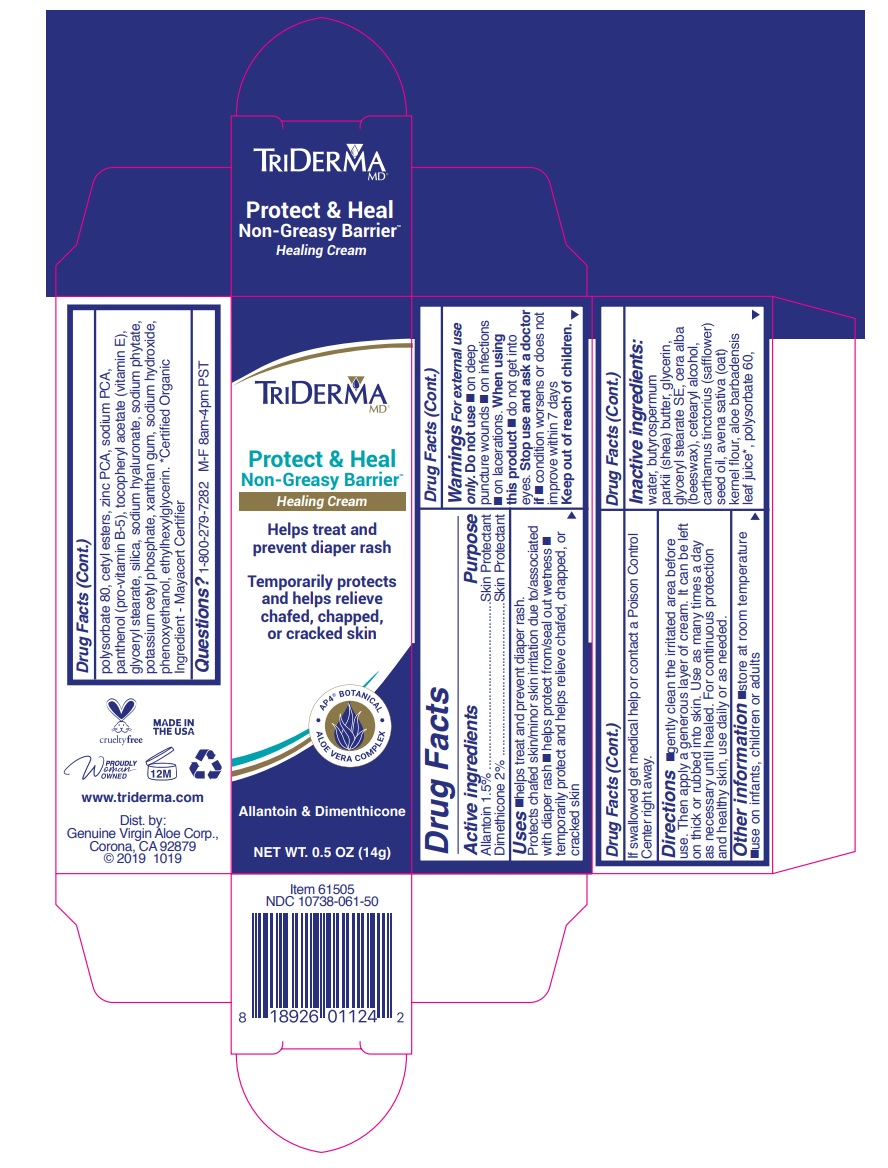
Label: TRIDERMA PROTECT AND HEAL NON- GREASY BARRIER- allantoin, dimethicone cream
- NDC Code(s): 10738-061-45, 10738-061-50, 10738-061-55
- Packager: Genuine Virgin Aloe Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- WARNINGS
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients: water, butyrospermum parkii (shea) butter, glycerin, glyceryl stearate SE, cera alba (beeswax), cetearyl alcohol, carthamus tinctorius (safflower) seed oil, avena sativa (oat) kernel flour, aloe barbadensis leaf juice*, polysorbate 60, polysorbate 80, cetyl esters, zinc PCA, sodium PCA, panthenol (pro-vitamin B-5), tocopheryl acetate (vitamin E), glyceryl stearate, silica, sodium hyaluronate, sodium phytate, potassium cetyl phosphate, xanthan gum, sodium hydroxide, phenoxyethanol, ethylhexylglycerin.*Certified Organic Ingredient - Mayacert Certifier
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- Packaging
- Packaging
- Packaging
-
INGREDIENTS AND APPEARANCE
TRIDERMA PROTECT AND HEAL NON- GREASY BARRIER
allantoin, dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10738-061 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 1.5 g in 100 g DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 2 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SHEA BUTTER (UNII: K49155WL9Y) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) YELLOW WAX (UNII: 2ZA36H0S2V) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SAFFLOWER OIL (UNII: 65UEH262IS) OATMEAL (UNII: 8PI54V663Y) ALOE VERA LEAF (UNII: ZY81Z83H0X) POLYSORBATE 60 (UNII: CAL22UVI4M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CETYL ESTERS WAX (UNII: D072FFP9GU) ZINC PIDOLATE (UNII: C32PQ86DH4) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) PANTHENOL (UNII: WV9CM0O67Z) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PHYTATE SODIUM (UNII: 88496G1ERL) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10738-061-45 113 g in 1 TUBE; Type 0: Not a Combination Product 08/30/2019 2 NDC:10738-061-50 1 in 1 CARTON 08/30/2019 2 NDC:10738-061-55 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 08/30/2019 Labeler - Genuine Virgin Aloe Corporation (961374147) Establishment Name Address ID/FEI Business Operations Genuine Virgin Aloe Corporation 961374147 manufacture(10738-061)