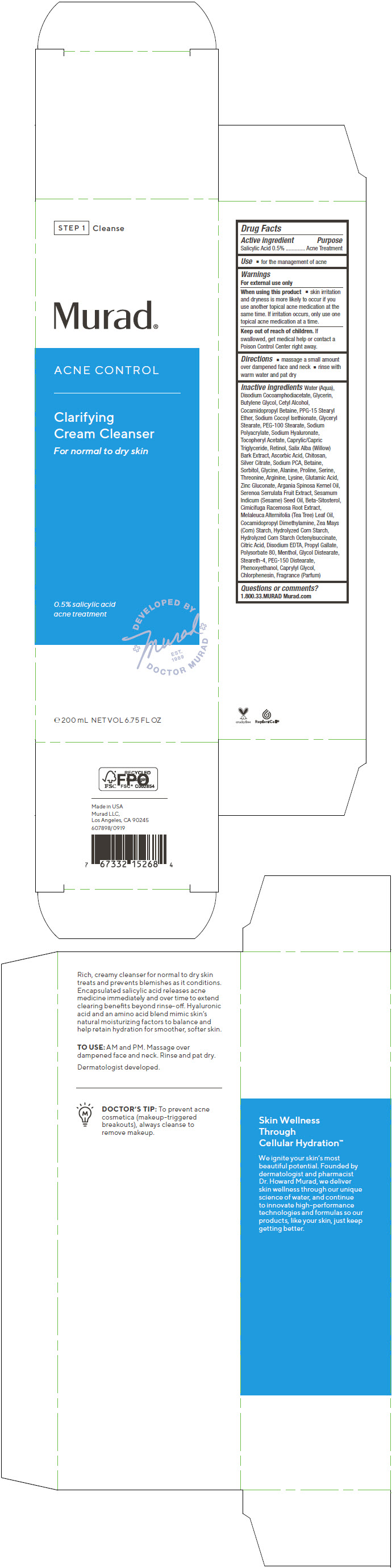
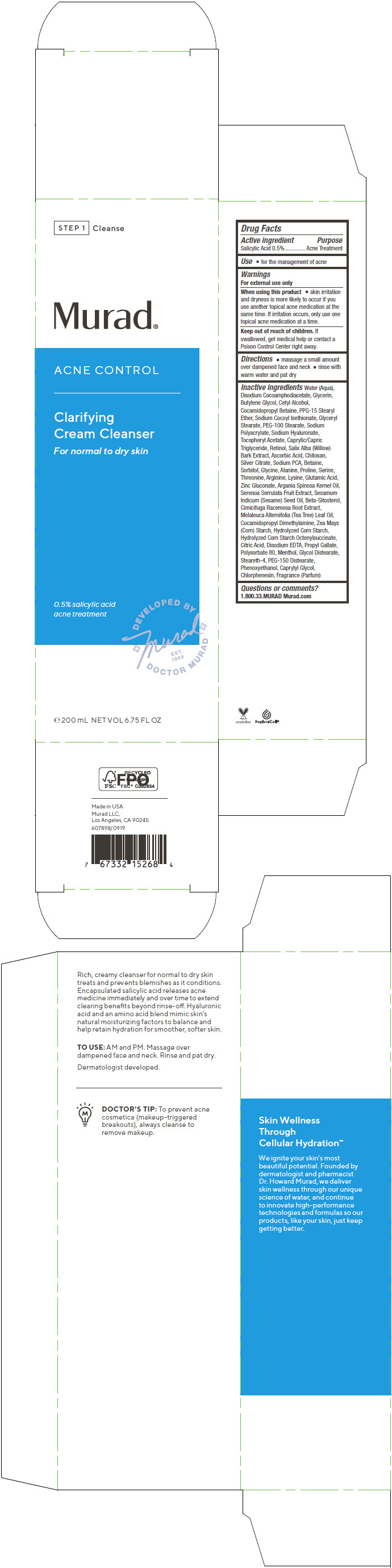
Label: ACNE CONTROL CLARIFYING CREAM CLEANSER- salicylic acid cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 70381-114-01, 70381-114-02 - Packager: Murad, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 2, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
-
Inactive ingredients
Water (Aqua), Disodium Cocoamphodiacetate, Glycerin, Butylene Glycol, Cetyl Alcohol, Cocamidopropyl Betaine, PPG-15 Stearyl Ether, Sodium Cocoyl Isethionate, Glyceryl Stearate, PEG-100 Stearate, Sodium Polyacrylate, Sodium Hyaluronate, Tocopheryl Acetate, Caprylic/Capric Triglyceride, Retinol, Salix Alba (Willow) Bark Extract, Ascorbic Acid, Chitosan, Silver Citrate, Sodium PCA, Betaine, Sorbitol, Glycine, Alanine, Proline, Serine, Threonine, Arginine, Lysine, Glutamic Acid, Zinc Gluconate, Argania Spinosa Kernel Oil, Serenoa Serrulata Fruit Extract, Sesamum Indicum (Sesame) Seed Oil, Beta-Sitosterol, Cimicifuga Racemosa Root Extract, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Cocamidopropyl Dimethylamine, Zea Mays (Corn) Starch, Hydrolyzed Corn Starch, Hydrolyzed Corn Starch Octenylsuccinate, Citric Acid, Disodium EDTA, Propyl Gallate, Polysorbate 80, Menthol, Glycol Distearate, Steareth-4, PEG-150 Distearate, Phenoxyethanol, Caprylyl Glycol, Chlorphenesin, Fragrance (Parfum)
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 200 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
ACNE CONTROL CLARIFYING CREAM CLEANSER
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70381-114 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETYL ALCOHOL (UNII: 936JST6JCN) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) PPG-15 STEARYL ETHER (UNII: 1II18XLS1L) SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) RETINOL (UNII: G2SH0XKK91) SALIX ALBA BARK (UNII: 205MXS71H7) ASCORBIC ACID (UNII: PQ6CK8PD0R) POLIGLUSAM (UNII: 82LKS4QV2Y) SILVER CITRATE (UNII: CKA421A1J7) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) BETAINE (UNII: 3SCV180C9W) SORBITOL (UNII: 506T60A25R) GLYCINE (UNII: TE7660XO1C) ALANINE (UNII: OF5P57N2ZX) PROLINE (UNII: 9DLQ4CIU6V) SERINE (UNII: 452VLY9402) THREONINE (UNII: 2ZD004190S) ARGININE (UNII: 94ZLA3W45F) LYSINE (UNII: K3Z4F929H6) GLUTAMIC ACID (UNII: 3KX376GY7L) ZINC GLUCONATE (UNII: U6WSN5SQ1Z) ARGAN OIL (UNII: 4V59G5UW9X) SAW PALMETTO (UNII: J7WWH9M8QS) SESAME OIL (UNII: QX10HYY4QV) .BETA.-SITOSTEROL (UNII: S347WMO6M4) BLACK COHOSH (UNII: K73E24S6X9) TEA TREE OIL (UNII: VIF565UC2G) COCAMIDOPROPYL DIMETHYLAMINE (UNII: L36BM7DG2T) STARCH, CORN (UNII: O8232NY3SJ) CORN SYRUP (UNII: 9G5L16BK6N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PROPYL GALLATE (UNII: 8D4SNN7V92) POLYSORBATE 80 (UNII: 6OZP39ZG8H) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) GLYCOL DISTEARATE (UNII: 13W7MDN21W) STEARETH-4 (UNII: 1VBG09S3UL) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) Product Characteristics Color WHITE (Off-White) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70381-114-02 1 in 1 CARTON 07/28/2021 1 NDC:70381-114-01 200 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 07/28/2021 Labeler - Murad, LLC (781254792) Establishment Name Address ID/FEI Business Operations Cosway Company, Inc. 052400223 MANUFACTURE(70381-114)