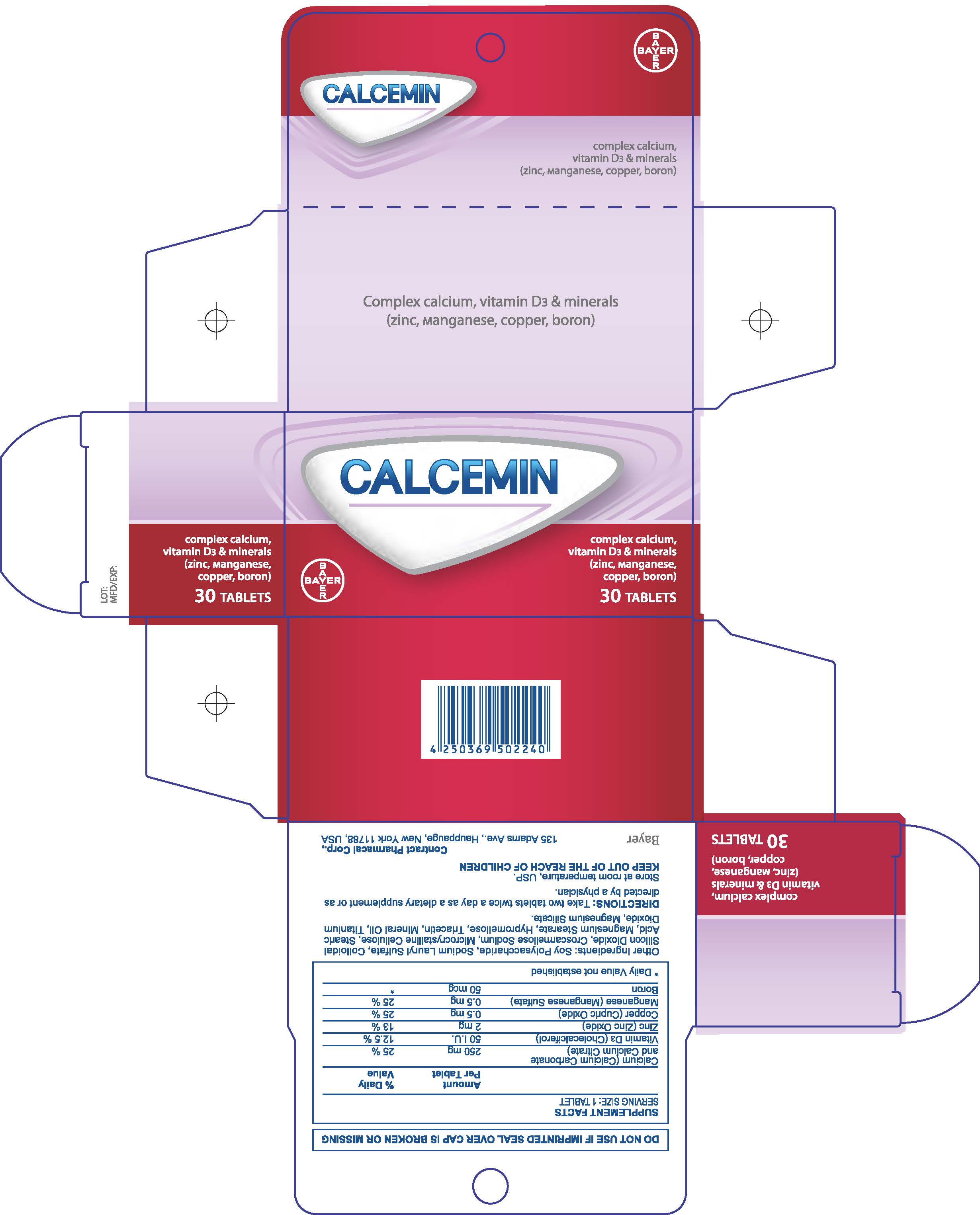
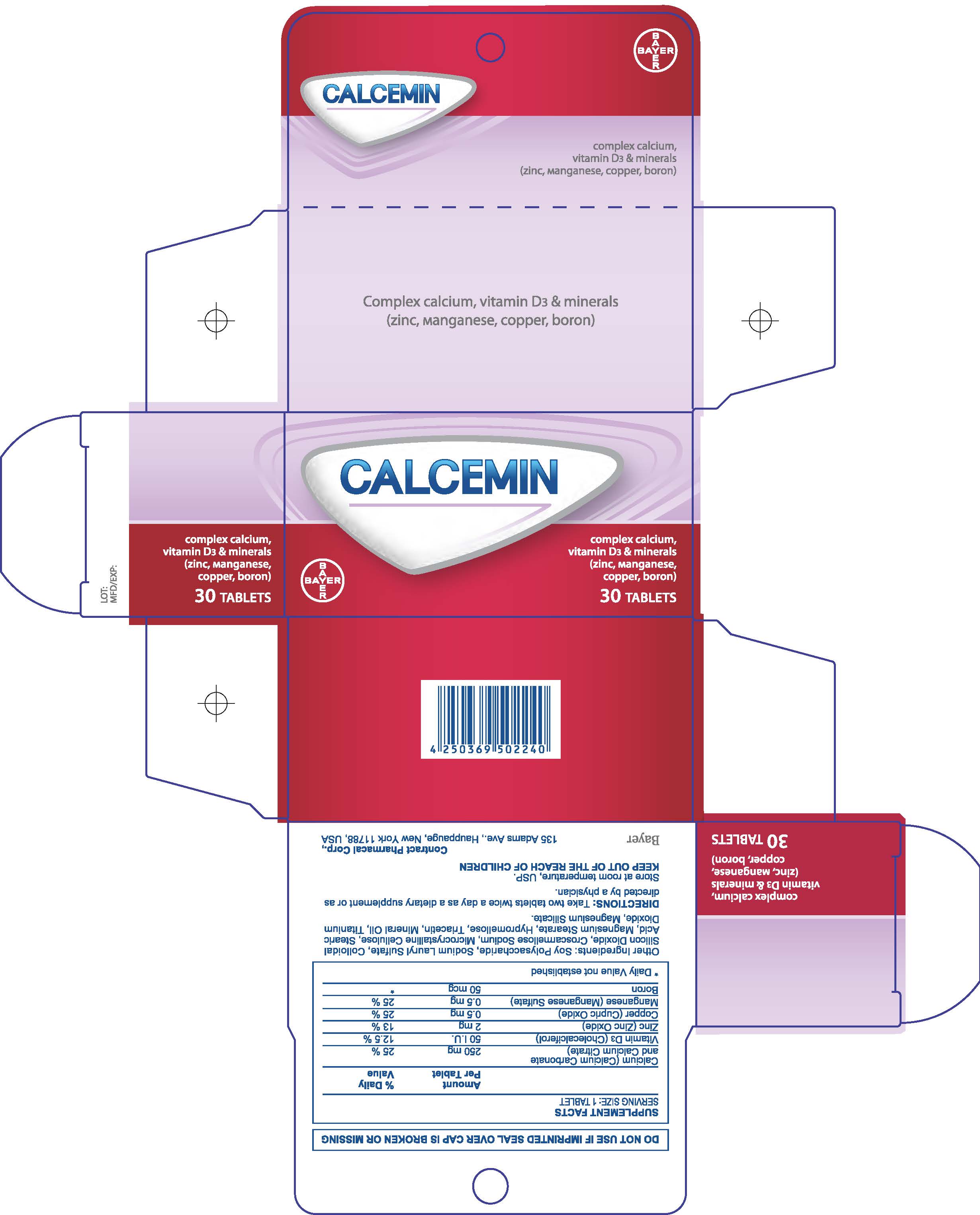
Label: CALCEMIN- calcium carbonate tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 0280-0271-12, 0280-0271-30, 0280-0271-60 - Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated January 9, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- 30 Count Carton
-
INGREDIENTS AND APPEARANCE
CALCEMIN
calcium carbonate tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0280-0271 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PREVITAMIN D3 (UNII: HDA46400N5) (PREVITAMIN D3 - UNII:HDA46400N5) PREVITAMIN D3 0.75 mg ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 2 mg BORON (UNII: N9E3X5056Q) (BORON - UNII:N9E3X5056Q) BORON 50 ug Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSES (UNII: 3NXW29V3WO) TRIACETIN (UNII: XHX3C3X673) MINERAL OIL (UNII: T5L8T28FGP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MAGNESIUM SILICATE (UNII: 9B9691B2N9) Product Characteristics Color white Score no score Shape OVAL Size 15mm Flavor Imprint Code None Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0280-0271-30 1 in 1 CARTON 04/01/2016 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:0280-0271-60 1 in 1 CARTON 04/01/2016 2 60 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:0280-0271-12 1 in 1 CARTON 04/01/2016 3 120 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 04/01/2016 Labeler - Bayer HealthCare LLC. (112117283) Establishment Name Address ID/FEI Business Operations Contract Pharmacal 968335112 manufacture(0280-0271) , pack(0280-0271)