Label: SUDAFED SINUS 12 HOUR PRESSURE PLUS PAIN- naproxen sodium and pseudoephedrine hydrochloride tablet, multilayer, extended release
- NDC Code(s): 50580-434-01
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Allergy alert
Naproxen sodium may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Heart attack and stroke warning
NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- in children under 12 years of age
Ask a doctor before use if
- the stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, thyroid disease, diabetes, have trouble urinating due to an enlarged prostate gland, or had a stroke
- you are taking a diuretic
- you have problems or serious side effects from taking pain relievers or fever reducers
Ask a doctor or pharmacist before use if you are
- under a doctor's care for any serious condition
- taking aspirin for heart attack or stroke, because naproxen may decrease this benefit of aspirin
- taking any other drug
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke:
- chest pain
- trouble breathing
- weakness in one part or side of body
- slurred speech
- leg swelling
- redness or swelling is present in the painful area
- any new symptoms appear
- fever gets worse or lasts more than 3 days
- you have difficulty swallowing or the caplet feels stuck in your throat
- you get nervous, dizzy, or sleepless
- nasal congestion lasts more than 7 days
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
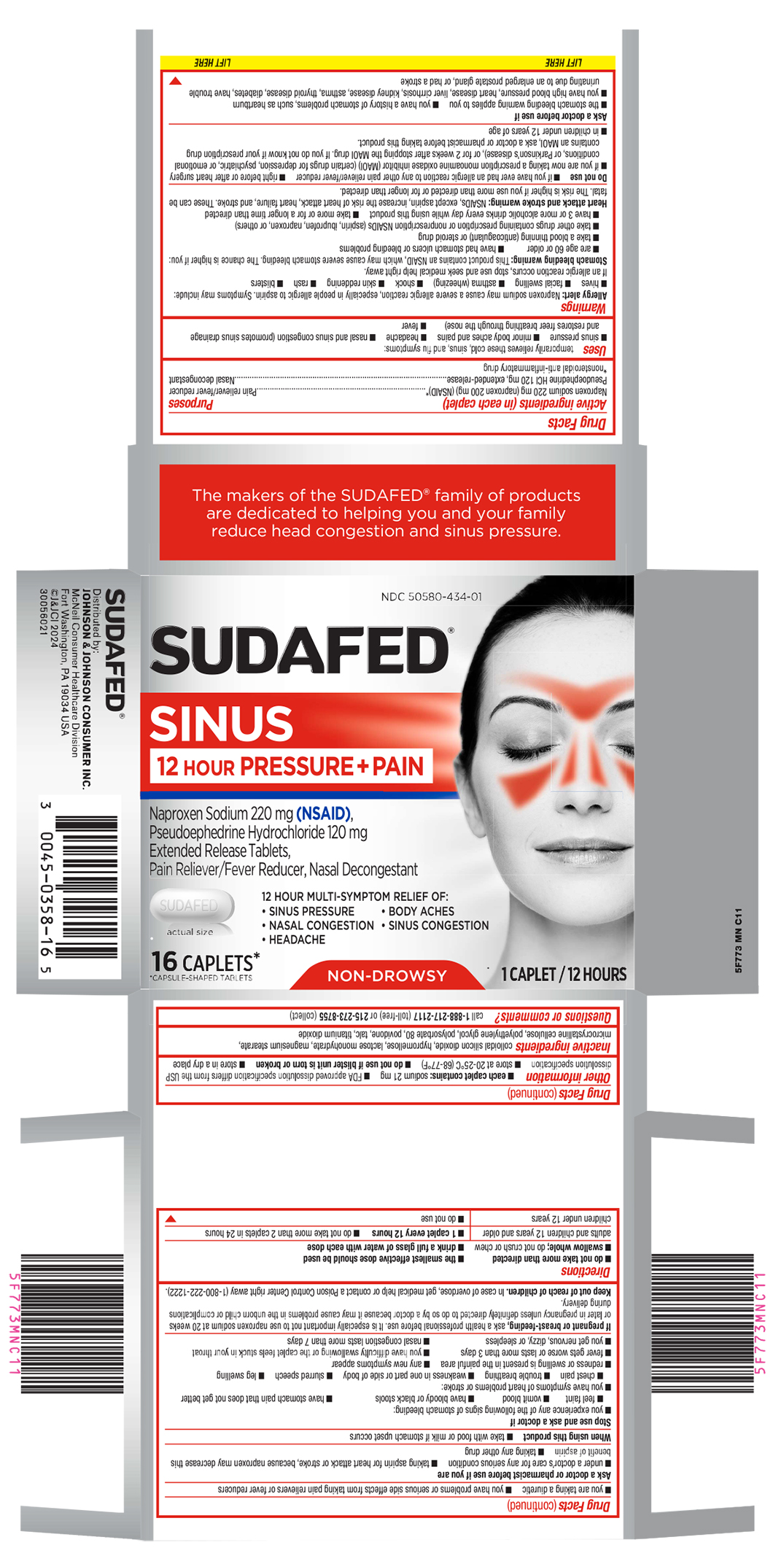
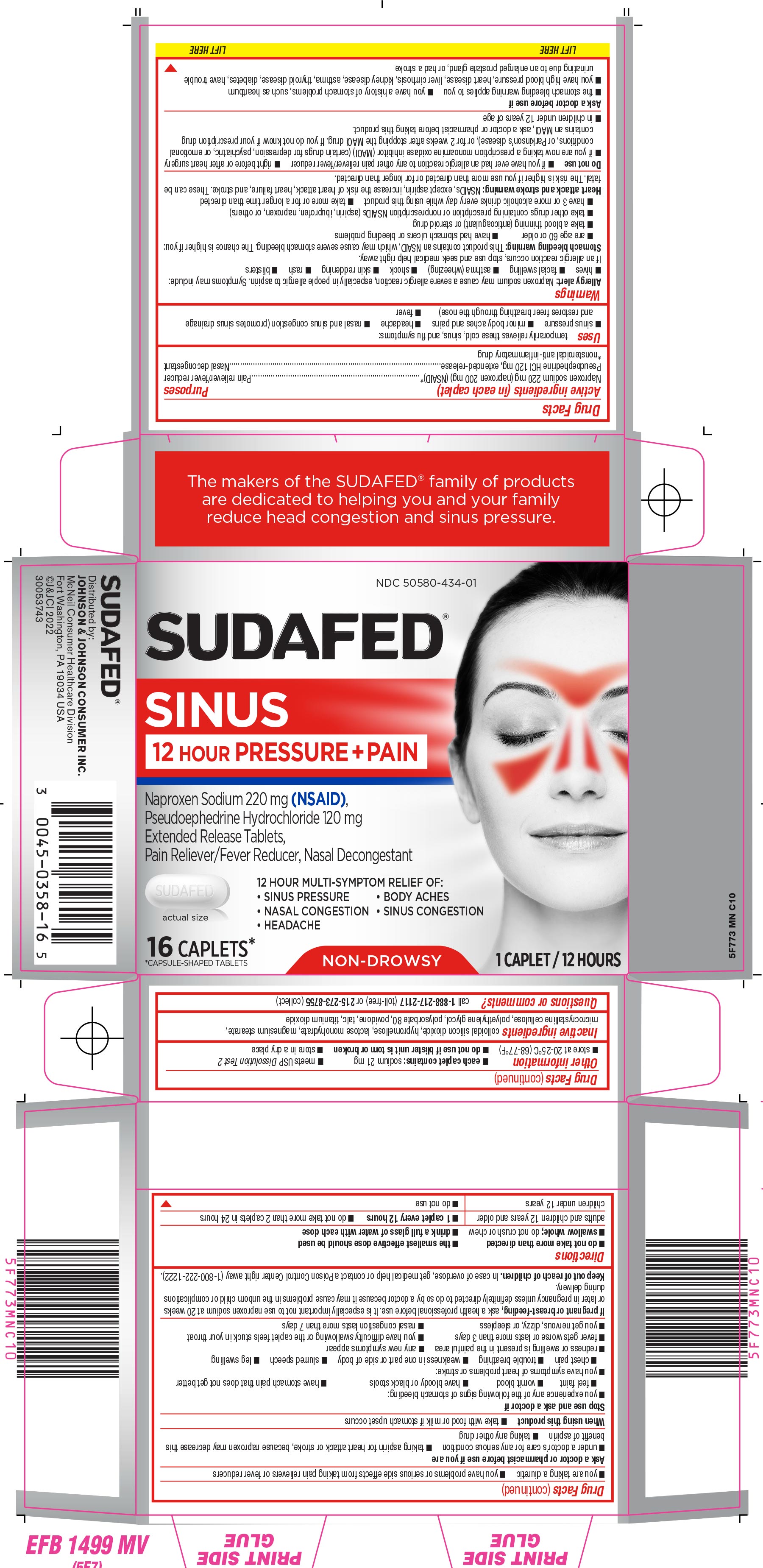
PRINCIPAL DISPLAY PANEL
NDC 50580-434-01
SUDAFED ®
SINUS
12 HOUR PRESSURE + PAINNaproxen Sodium 220 mg (NSAID),
Pseudoephedrine Hydrochloride 120 mg
Extended Release Tablets,
Pain Reliever/Fever Reducer, Nasal Decongestant12 HOUR MULTI-SYMPTOM RELIEF OF:
• SINUS PRESSURE • BODY ACHES
• NASAL CONGESTION • SINUS CONGESTION
• HEADACHEactual size
16 CAPLETS*
*CAPSULE-SHAPED TABLETSNON-DROWSY
1 CAPLET / 12 HOURS
-
INGREDIENTS AND APPEARANCE
SUDAFED SINUS 12 HOUR PRESSURE PLUS PAIN
naproxen sodium and pseudoephedrine hydrochloride tablet, multilayer, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50580-434 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPROXEN SODIUM (UNII: 9TN87S3A3C) (NAPROXEN - UNII:57Y76R9ATQ) NAPROXEN SODIUM 220 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 120 mg Inactive Ingredients Ingredient Name Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color white Score no score Shape OVAL Size 19mm Flavor Imprint Code SUDAFED Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50580-434-01 2 in 1 CARTON 06/17/2019 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076518 06/17/2019 Labeler - Kenvue Brands LLC (118772437)