Label: CHILDREN ALLERGY- fexofenadine hcl suspension
- NDC Code(s): 0363-2600-04, 0363-2600-08
- Packager: Walgreens
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
-
Direction
- shake well before using
- use only with enclosed dosing cup
adults and children 12 years of age and over take 10 mL every 12 hours; do not take more than 20 mL in 24 hours children 2 to under 12 years of age take 5 mL every 12 hours; do not take more than 10 mL in 24 hours children under 2 years of age ask a doctor adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor Note: mL = milliliters
- Other information
- Inactive ingredients
- Questions or comments?
-
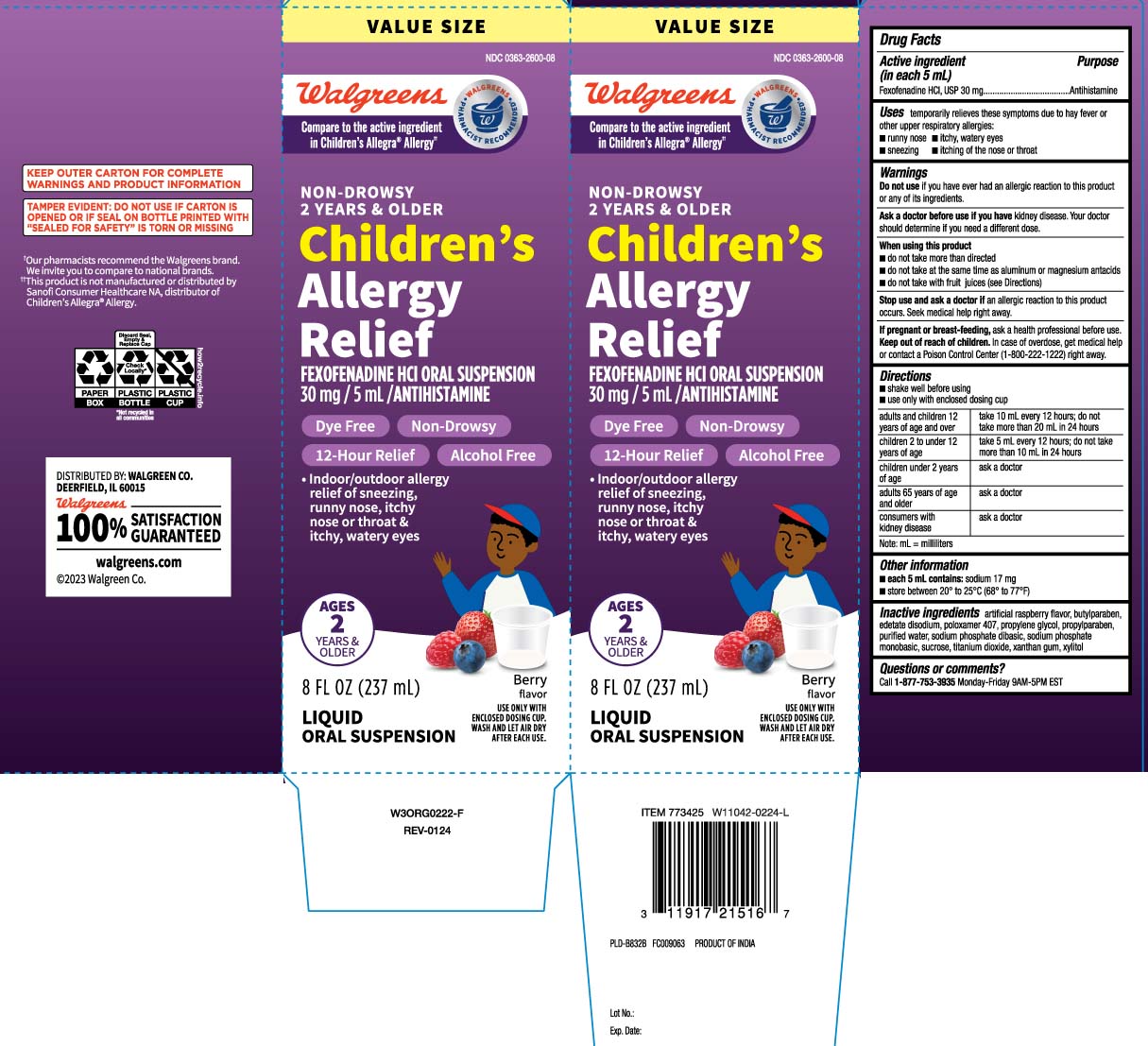
Principal Display Panel
Compare to the active ingredient in Children's Allegra® Allergy††
NON-DROWSY
2 YEARS & OLDER
Children's Allergy Relief
FEXOFENADINE HCl ORAL SUSPENSION
30 mg/ 5 mL/ ANTIHISTAMINE
Dye Free Non-Drowsy
12-Hour Relief Alcohol Free
- Indoor/outdoor allergy relief of sneezing, runny nose, itchy nose or throat & itchy, watery eyes
AGES 2 YEARS & OLDER
Berry flavor
FL OZ (mL)
LIQUID ORAL SUSPENSION
USE ONLY WITH ENCLOSED DOSING CUP. WASH AND LET AIR DRY AFTER EACH USE
*This product is not manufactured or distributed by Sanofi Consumer Healthcare NA, distributor of Children's Allegra® Allergy.
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF SEAL ON BOTTLE PRINTED WITH "SEALED FOR SAFETY" ISTORN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION .
DISTRIBUTED BY: WALGREEN CO.
DEERFIELD, IL 60015
- Product Label
-
INGREDIENTS AND APPEARANCE
CHILDREN ALLERGY
fexofenadine hcl suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-2600 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength BUTYLPARABEN (UNII: 3QPI1U3FV8) EDETATE DISODIUM (UNII: 7FLD91C86K) POLOXAMER 407 (UNII: TUF2IVW3M2) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-2600-04 1 in 1 BOX 10/31/2023 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:0363-2600-08 1 in 1 BOX 10/31/2023 2 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203330 10/31/2023 Labeler - Walgreens (008965063)