Label: MAXIMUM STRENGTH MUCUS RELIEF COLD,FLU AND SORE THROAT- acetaminophen, dextromethorphan hydrobromide, guaifenesin ,phenylephrine hcl liquid
- NDC Code(s): 49035-771-09
- Packager: Wal-Mart Stores,Inc.,
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
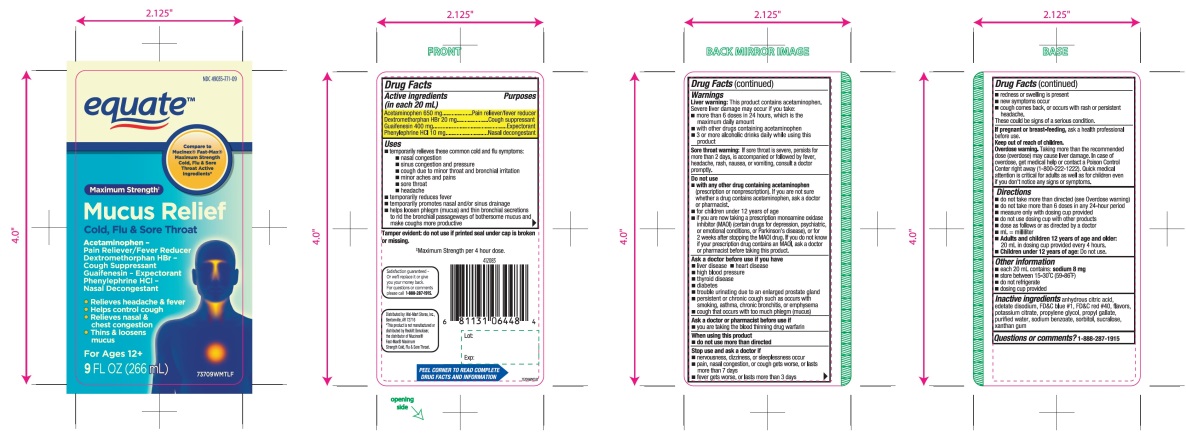
- ACTIVE INGREDIENT
-
Uses
- •
- temporarily relieves these common cold and flu symptoms:
- •
- nasal congestion
- •
- sinus congestion and pressure
- •
- cough due to minor throat and bronchial irritation
- •
- minor aches and pains
- •
- sore throat
- •
- headache
- •
- temporarily reduces fever
- •
- temporarily promotes nasal and/or sinus drainage
- •
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
-
Warnings
Liver warning:
This product contains acetaminophen. Severe liver damage may occur if you take:
- •
- more than 6 doses in 24 hours, which is the maximum daily amount
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks daily while using this product
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- for children under 12 years of age
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- liver disease
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
- •
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- •
- cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor if
- •
- nervousness, dizziness, or sleeplessness occur
- •
- pain, nasal congestion, or cough gets worse, or lasts more than 7 days
- •
- fever gets worse, or lasts more than 3 days
- •
- redness or swelling is present
- •
- new symptoms occur
- •
- cough comes back, or occurs with rash, or persistent headache.
- These could be signs of a serious condition.
- Overdose warning
-
Directions
- •
- do not take more than directed (see Overdose Warning))
- •
- do not take more than 6 doses in any 24-hour period
- •
- measure only with dosing cup provided
- •
- do not use dosing cup with other products
- •
- dose as follows or as directed by a doctor
- •
- mL= milliliter
- •
- Adults and children 12 years of age and older: 20 mL in dosing cup provided every 4 hours
- •
- Children under 12 years of age: Do not use
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
equate™
NDC 49035-771-09
Compare to Mucinex® Fast-Max® Maximum Strength Cold, Flu & Sore Throat Active Ingredients*
Maximum Strength‡
Mucus Relief
Cold, Flu & Sore Throat
Acetaminophen - Pain Reliever/Fever Reducer
Dextromethorphan HBr - Cough Suppressant
Guaifenesin - Expectorant
Phenylephrine HCl - Nasal Decongestant- •
- Relieves headache & fever
- •
- Helps control cough
- •
- Relieves nasal & chest congestion
- •
- Thins & loosens mucus
For Ages 12+
9FL OZ (266mL)
Tamper evident: do not use if printed seal under cap is broken or missing.
‡Maximum Strength per 4 hour dose.
Satisfaction guaranteed-Or we’ll replace it or give you your money back For questions or comments please call 1-888-287-1915
Distributed by: Wal-Mart Stores, Inc.,
Bentonville, AR 72716
*This product is not manufactured or distributed by Reckitt Benckiser, the distributor of Mucinex® Fast-Max® Maximum Strength Cold, Flu & Sore Throat.
-
INGREDIENTS AND APPEARANCE
MAXIMUM STRENGTH MUCUS RELIEF COLD,FLU AND SORE THROAT
acetaminophen, dextromethorphan hydrobromide, guaifenesin ,phenylephrine hcl liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-771 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 20 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg in 20 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) POTASSIUM CITRATE (UNII: EE90ONI6FF) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYL GALLATE (UNII: 8D4SNN7V92) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-771-09 266 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/06/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 09/06/2017 Labeler - Wal-Mart Stores,Inc., (051957769)