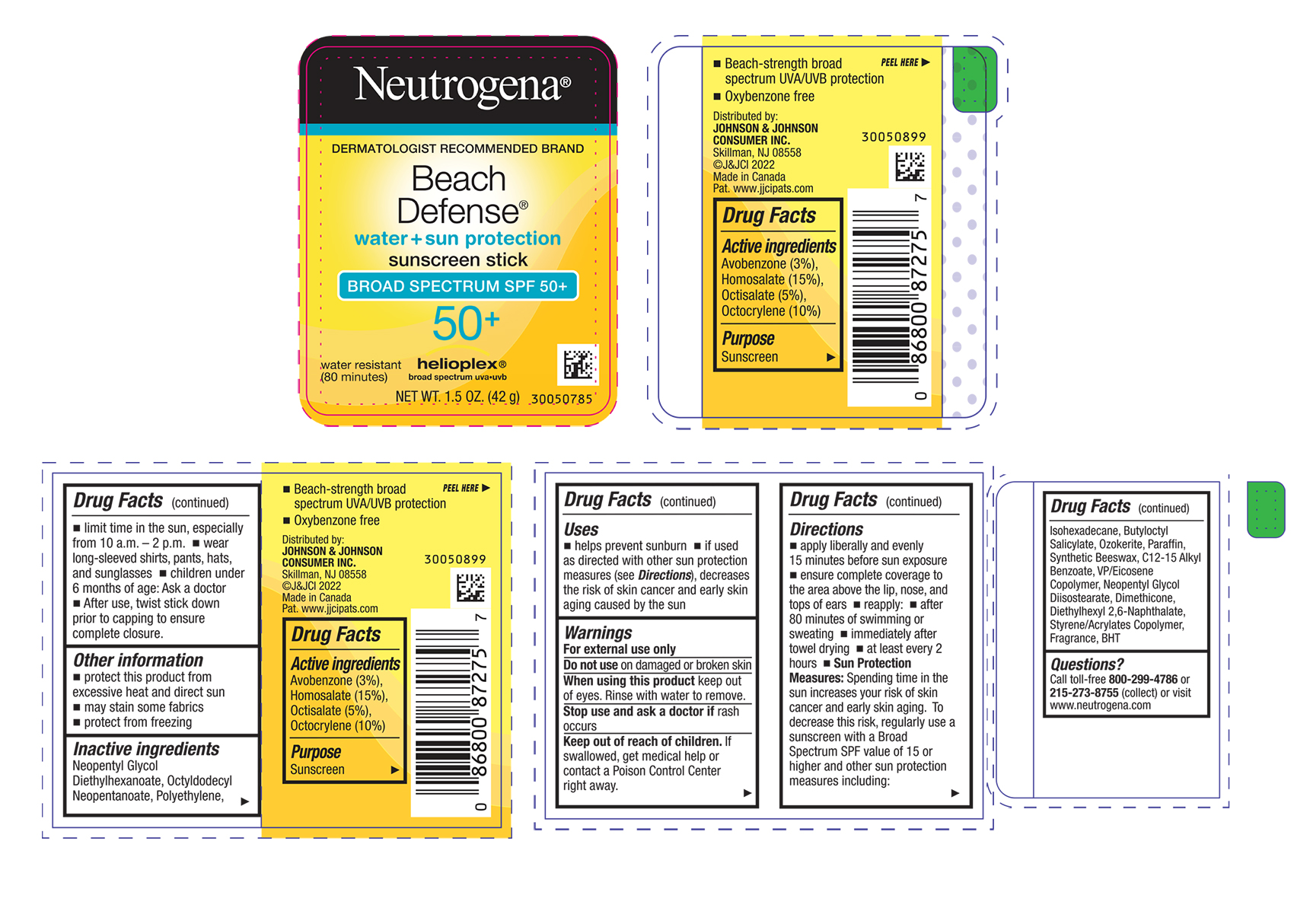
Label: NEUTROGENA BEACH DEFENSE WATER PLUS SUN PROTECTION SUNSCREEN STICK BROAD SPECTRUM SPF 50 PLUS- avobenzone, homosalate, octisalate, and octocrylene spray
- NDC Code(s): 69968-0574-2, 69968-0574-3
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally and evenly 15 minutes before sun exposure
- ensure complete coverage to the area above the lip, nose and tops of ears
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
- After use, twist stick down prior to capping to ensure complete closure.
- Other information
-
Inactive ingredients
Neopentyl Glycol Diethylhexanoate, Octyldodecyl Neopentanoate, Polyethylene, Isohexadecane, Butyloctyl Salicylate, Ozokerite, Paraffin, Synthetic Beeswax, C12-15 Alkyl Benzoate, VP/Eicosene Copolymer, Neopentyl Glycol Diisostearate, Dimethicone, Diethylhexyl 2,6-Naphthalate, Styrene/Acrylates Copolymer, Fragrance, BHT
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 42 g Canister Label
-
INGREDIENTS AND APPEARANCE
NEUTROGENA BEACH DEFENSE WATER PLUS SUN PROTECTION SUNSCREEN STICK BROAD SPECTRUM SPF 50 PLUS
avobenzone, homosalate, octisalate, and octocrylene sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0574 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 150 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 g Inactive Ingredients Ingredient Name Strength NEOPENTYL GLYCOL DIETHYLHEXANOATE (UNII: U68ZV6W62C) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) ISOHEXADECANE (UNII: 918X1OUF1E) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CERESIN (UNII: Q1LS2UJO3A) PARAFFIN (UNII: I9O0E3H2ZE) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) NEOPENTYL GLYCOL DIISOSTEARATE (UNII: 4M6OQ34JWW) DIMETHICONE (UNII: 92RU3N3Y1O) DIETHYLHEXYL 2,6-NAPHTHALATE (UNII: I0DQJ7YGXM) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) YELLOW WAX (UNII: 2ZA36H0S2V) EICOSYL POVIDONE (UNII: XQQ9MKE2BJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0574-2 42 g in 1 CANISTER; Type 0: Not a Combination Product 10/07/2019 2 NDC:69968-0574-3 2 in 1 CARTON 10/07/2019 2 42 g in 1 CANISTER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/07/2019 Labeler - Johnson & Johnson Consumer Inc. (118772437)