Label: PFIZERPEN- penicillin g potassium powder, for solution
- NDC Code(s): 0049-0420-05, 0049-0420-10, 0049-0430-20
- Packager: Roerig
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Penicillin G Potassium and other antibacterial drugs, Buffered Pfizerpen (Penicillin G Potassium for Injection, USP) should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
-
DESCRIPTION
Buffered Pfizerpen (Penicillin G Potassium for Injection, USP) is a sterile, pyrogen-free powder for reconstitution. Buffered Pfizerpen for Injection is an antibacterial agent for intramuscular, continuous intravenous infusion, intrapleural or other local infusion, and intrathecal administration.
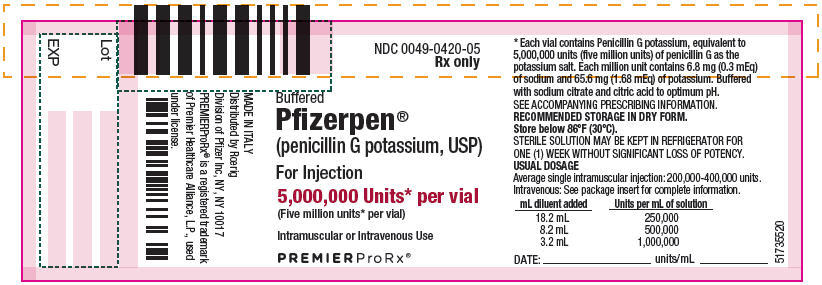
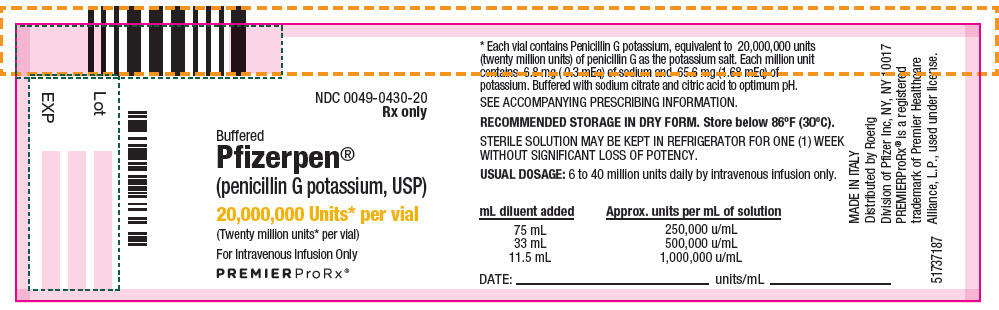
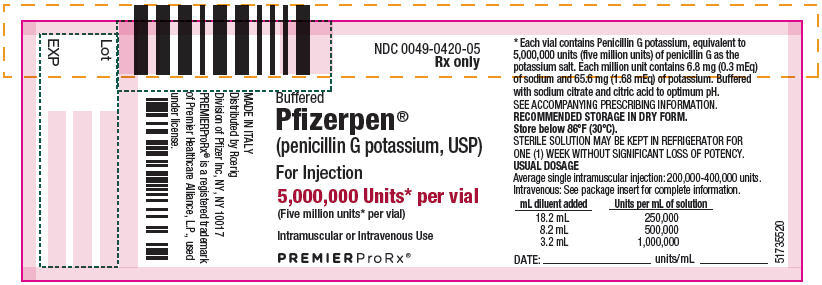
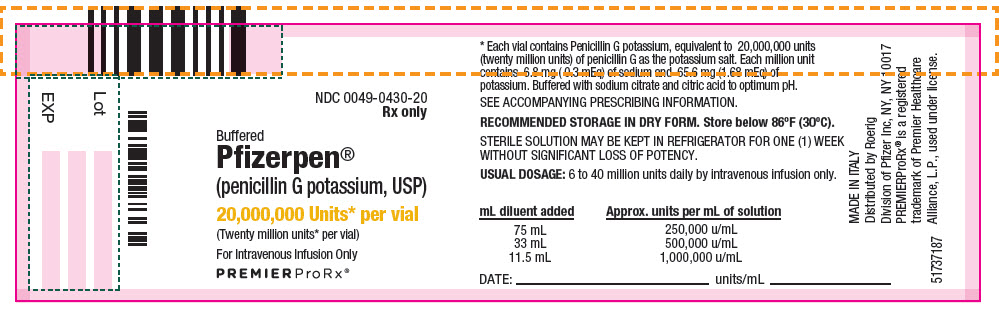
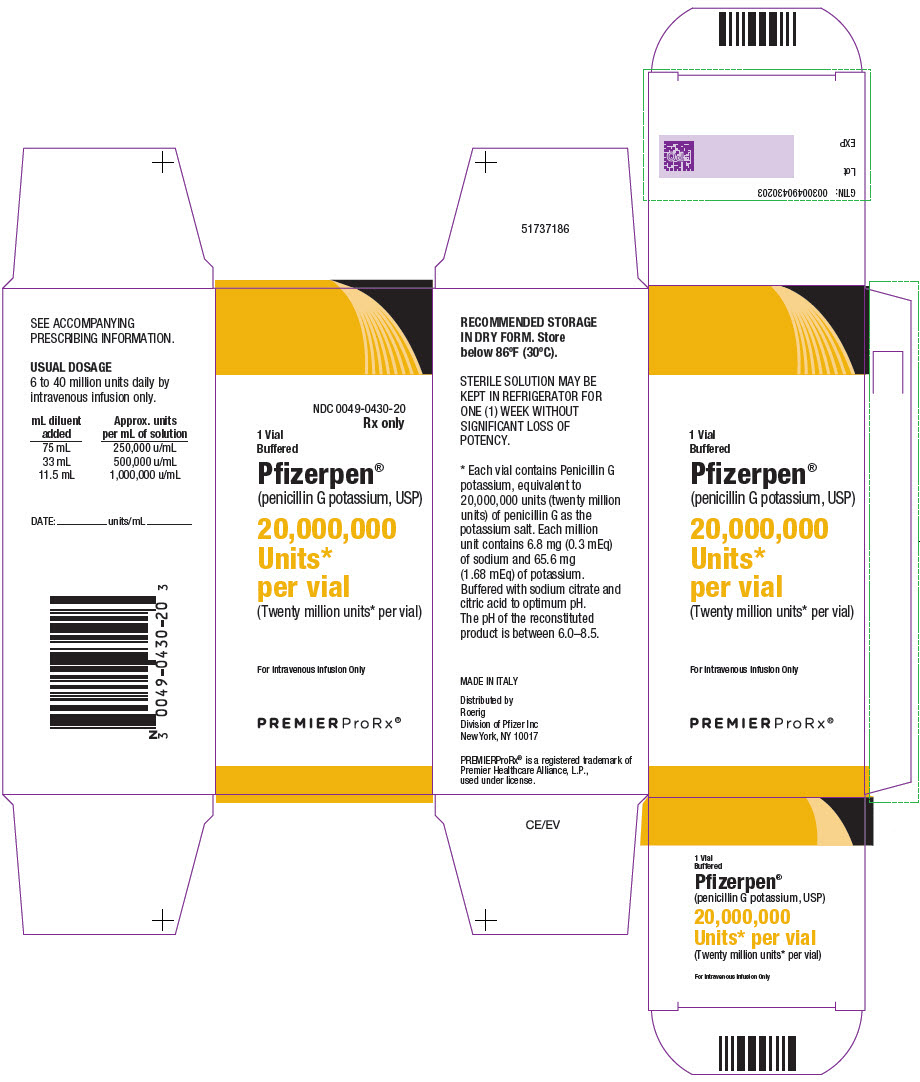
Each million units contains approximately 6.8 milligrams of sodium (0.3 mEq) and 65.6 milligrams of potassium (1.68 mEq). Buffered Pfizerpen (Penicillin G Potassium for Injection, USP) is supplied in vials equivalent to 5,000,000 units (5 million units) or 20,000,000 units (20 million units) of penicillin G as the potassium salt.
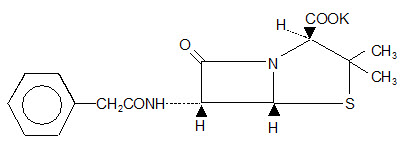
Chemically, Pfizerpen is monopotassium 3,3-dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo (3.2.0) heptane-2-carboxylate. It has a molecular weight of 372.48 and the following chemical structure:
Penicillin G potassium is a colorless or white crystal, or a white crystalline powder which is odorless, or practically so, and moderately hygroscopic. Penicillin G potassium is very soluble in water. The pH of the reconstituted product is between 6.0–8.5.
-
CLINICAL PHARMACOLOGY
Aqueous penicillin G is rapidly absorbed following both intramuscular and subcutaneous injection. Initial blood levels following parenteral administration are high but transient. Penicillins bind to serum proteins, mainly albumin. Therapeutic levels of the penicillins are easily achieved under normal circumstances in extracellular fluid and most other body tissues. Penicillins are distributed in varying degrees into pleural, pericardial, peritoneal, ascitic, synovial, and interstitial fluids. Penicillins are excreted in breast milk. Penetration into the cerebrospinal fluid, eyes, and prostate is poor. Penicillins are rapidly excreted in the urine by glomerular filtration and active tubular secretion, primarily as unchanged drug. Approximately 60 percent of the total dose of 300,000 units is excreted in the urine within this 5-hour period. For this reason, high and frequent doses are required to maintain the elevated serum levels desirable in treating certain severe infections in individuals with normal kidney function. In neonates and young infants, and in individuals with impaired kidney function, excretion is considerably delayed.
After an intravenous infusion of penicillin G, peak serum concentrations are attained immediately after completion of the infusion. In a study of ten patients administered a single 5 million unit dose of penicillin G intravenously over 3–5 minutes, the mean serum concentrations were 400 mcg/mL, 273 mcg/mL and 3.0 mcg/mL at 5–6 minutes, 10 minutes and 4 hours after completion of the injection, respectively. In a separate study, five healthy adults were administered one million units of penicillin G intravenously, either as a bolus over 4 minutes or as an infusion over 60 minutes. The mean serum concentration eight minutes after completion of the bolus was 45 mcg/mL and eight minutes after completion of the infusion was 14.4 mcg/mL. The mean β-phase serum half-life of penicillin G administered by the intravenous route in ten patients with normal renal function was 42 minutes, with a range of 31–50 minutes.
The clearance of penicillin G in normal individuals is predominantly via the kidney. The renal clearance, which is extremely rapid, is the result of glomerular filtration and active tubular transport, with the latter route predominating. Urinary recovery is reported to be 58–85% of the administered dose. Renal clearance of penicillin is delayed in premature infants, neonates and in the elderly due to decreased renal function. The serum half-life of penicillin G correlates inversely with age and clearance of creatinine and ranges from 3.2 hours in infants 0 to 6 days of age to 1.4 hours in infants 14 days of age or older.
Nonrenal clearance includes hepatic metabolism and, to a lesser extent, biliary excretion. The latter routes become more important with renal impairment.
Probenecid blocks the renal tubular secretion of penicillin. Therefore, the concurrent administration of probenecid prolongs the elimination of penicillin G and, consequently, increases serum concentrations.
Penicillin G is distributed to most areas of the body including lung, liver, kidney, muscle, bone and placenta. In the presence of inflammation, levels of penicillin in abscesses, middle ear, pleural, peritoneal and synovial fluids are sufficient to inhibit most susceptible bacteria. Penetration in the eye, brain, cerebrospinal fluid (CSF) or prostate is poor in the absence of inflammation. With inflamed meninges, the penetration of penicillin G into the CSF improves, such that the CSF/serum ratio is 2–6%. Inflammation also enhances its penetration into the pericardial fluid. Penicillin G is actively secreted into the bile resulting in levels at least 10 times those achieved simultaneously in serum. Penicillin G penetrates poorly into human polymorphonuclear leukocytes.
In the presence of impaired renal function, the β-phase serum half-life of penicillin G is prolonged. β-phase serum half-lives of one to two hours were observed in azotemic patients with serum creatinine concentrations <3 mg/100 mL and ranged as high as 20 hours in anuric patients. A linear relationship, including the lowest range of renal function, is found between the serum elimination rate constant and renal function as measured by creatinine clearance.
In patients with altered renal function, the presence of hepatic insufficiency further alters the elimination of penicillin G. In one study, the serum half-lives in two anuric patients (excreting <400 mL urine/day) were 7.2 and 10.1 hours. A totally anuric patient with terminal hepatic cirrhosis had a penicillin half-life of 30.5 hours, while another patient with anuria and liver disease had a serum half-life of 16.4 hours. The dosage of penicillin G should be reduced in patients with severe renal impairment, with additional modifications when hepatic disease accompanies the renal impairment. Hemodialysis has been shown to reduce penicillin G serum levels.
Microbiology
Penicillin G exerts a bactericidal action against penicillin-susceptible microorganisms during the stage of active multiplication. It acts through the inhibition of biosynthesis of cell wall peptidoglycan rendering the cell wall osmotically unstable. It is not active against the penicillinase-producing bacteria, which include many strains of staphylococci or against organisms resistant to beta-lactams because of alterations in the penicillin-binding proteins, such as methicillin-resistant staphylococci. Penicillin G is highly active in vitro against streptococci (groups A, B, C, G, H, L and M) and Neisseria meningitidis.
Other organisms susceptible to penicillin G are N. gonorrhoeae, Corynebacterium diphtheriae, Bacillus anthracis, Clostridium spp., Actinomyces species, "Spirillum minus", Streptobacillus moniliformis, Listeria monocytogenes and Leptospira spp.; Treponema pallidum is extremely sensitive to the bactericidal action of penicillin G.
Some species of gram-negative bacilli were previously considered susceptible to very high intravenous doses of penicillin G (up to 80 million units/day) including some strains of Escherichia coli, Proteus mirabilis, Salmonella spp. and Shigella spp.; Enterobacter aerogenes (formerly Aerobacter aerogenes) and Alcaligenes faecalis. Penicillin G is no longer considered a drug of choice for infections caused by these organisms.
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
INDICATIONS AND USAGE
Therapy
Penicillin G Potassium for Injection, USP is indicated in the treatment of serious infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
Appropriate culture and susceptibility tests should be done before treatment in order to isolate and identify organisms causing infection and to determine their susceptibility to penicillin G.
Therapy with Penicillin G Potassium for Injection, USP may be initiated before results of such tests are known when there is reason to believe the infection may involve any of the organisms listed below; however, once these results become available, appropriate therapy should be continued.
CLINICAL INDICATION INFECTING ORGANISM Septicemia, empyema, pneumonia, pericarditis, endocarditis, meningitis
Streptococcus pyogenes (group A β-hemolytic streptococcus), other β-hemolytic streptococci including groups C, H, G, L and M, Streptococcus pneumoniae and Staphylococcus species (non-penicillinase producing strains)
Anthrax
Bacillus anthracis
Actinomycosis (cervico-facial disease and thoracic and abdominal disease)
Actinomyces israelii
Botulism (adjunctive therapy to antitoxin), gas gangrene, and tetanus (adjunctive therapy to human tetanus immune globulin)
Clostridium species
Diphtheria (adjunctive therapy to antitoxin and prevention of the carrier state)
Corynebacterium diphtheriae
Erysipelothrix endocarditis
Erysipelothrix rhusiopathiae
Fusospirochetosis (severe infections of the oropharynx [Vincent's], lower respiratory tract and genital area)
Fusobacterium species and spirochetes
Listeria infections including meningitis and endocarditis
Listeria monocytogenes
Pasteurella infections including bacteremia and meningitis
Pasteurella multocida
Haverhill fever
Streptobacillus moniliformis
Rat bite fever
Spirillum minus or Streptobacillus moniliformis
Disseminated gonococcal infections
Neisseria gonorrhoeae (Penicillin-susceptible)
Syphilis (congenital and neurosyphilis)
Treponema pallidum
Meningococcal meningitis and/or septicemia
Neisseria meningitidis
Gram-negative bacillary infections (bacteremias)
Penicillin G is not the drug of choice in the treatment of Gram-negative bacillary infections.Gram-negative bacillary organisms (i.e. Enterobacteriaceae)
To reduce the development of drug-resistant bacteria and maintain effectiveness of Penicillin G Potassium and other antibacterial drugs, Penicillin G Potassium should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- CONTRAINDICATIONS
-
WARNINGS
Anaphylaxis
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on penicillin therapy. These reactions are more likely to occur in individuals with a history of penicillin hypersensitivity and/or a history of sensitivity to multiple allergens. There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe reactions when treated with cephalosporins. Before initiating therapy with penicillin G, careful inquiry should be made concerning previous hypersensitivity reactions to penicillins, cephalosporins, or other allergens. If an allergic reaction occurs, penicillin G should be discontinued and appropriate therapy instituted.
Severe cutaneous adverse reactions
Severe cutaneous adverse reactions (SCAR), such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported in patients taking beta-lactam antibiotics. When SCAR is suspected, Penicillin G Potassium for Injection, USP should be discontinued immediately and an alternative treatment should be considered.
Clostridioides difficile associated diarrhea
Clostridioides difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Penicillin G Potassium for Injection, USP, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
PRECAUTIONS
General
Penicillin should be used with caution in individuals with histories of significant allergies and/or asthma (see Warnings). Whenever allergic reactions occur, penicillin should be withdrawn unless, in the opinion of the physician, the condition being treated is life-threatening and amenable only to penicillin therapy. Penicillin G Potassium for Injection, USP by the intravenous route in high doses (above 10 million units) should be administered slowly because of the potential adverse effects of electrolyte imbalance from the potassium content of the penicillin. Penicillin G Potassium for Injection, USP contains 1.68 mEq potassium and 0.3 mEq of sodium per million units. The use of antibiotics may promote overgrowth of nonsusceptible organisms, including fungi. Indwelling intravenous catheters encourage superinfections. Should superinfection occur, appropriate measures should be taken. When indicated, incision and drainage or other surgical procedures should be performed in conjunction with antibiotic therapy.
Prescribing Penicillin G Potassium for Injection, USP in the absence of proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Intramuscular and Intravenous Therapy
Care should be taken to avoid intravenous or accidental intraarterial administration, or injection into or near major peripheral nerves or blood vessels, since such injections may produce neurovascular damage. Particular care should be taken with IV administration because of the possibility of thrombophlebitis.
Information for Patients
Patients should be counseled that antibacterial drugs including Penicillin G Potassium for Injection, USP should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Penicillin G Potassium for Injection, USP is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Penicillin G Potassium for Injection, USP or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Laboratory Tests
Periodic assessment of organ system function, including frequent evaluation of electrolyte balance, hepatic, renal and hematopoietic systems, and cardiac and vascular status should be performed during prolonged therapy with high doses of intravenous penicillin G (see Adverse Reactions). If any impairment of function is suspected or known to exist, a reduction in the total dosage should be considered (see Dosage and Administration). In suspected staphylococcal infections, proper laboratory studies, including susceptibility tests should be performed. All infections due to Group A beta-hemolytic streptococci should be treated for at least 10 days.
Patients being treated for gonococcal infection should have a serologic test for syphilis before receiving penicillin. All cases of penicillin treated syphilis should receive adequate follow-up including clinical and serological examinations. The recommended follow-up varies with the stage of syphilis being treated.
Drug Interactions
Bacteriostatic antibacterials (i.e., chloramphenicol, erythromycins, sulfonamides or tetracyclines) may antagonize the bactericidal effect of penicillin, and concurrent use of these drugs should be avoided. This has been documented in vitro; however, the clinical significance of this interaction is not well-documented.
Penicillin blood levels may be prolonged by concurrent administration of probenecid which blocks the renal tubular secretion of penicillins. Other drugs may compete with penicillin G for renal tubular secretion and thus prolong the serum half-life of penicillin. These drugs include: aspirin, phenylbutazone, sulfonamides, indomethacin, thiazide diuretics, furosemide and ethacrynic acid.
Drug/Laboratory Test Interactions
After treatment with penicillin G, a false-positive reaction for glucose in the urine may occur with Benedict's solution, Fehling's solution or Clinitest® tablet, but not with the enzyme-based tests, such as Clinistix® and Tes-Tape®.
Penicillin G has been associated with pseudoproteinuria by certain test methods.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long term animal studies have been conducted with this drug.
Pregnancy
Teratogenic Effects
Reproduction studies performed in the mouse, rat, and rabbit have revealed no evidence of impaired fertility or harm to the fetus due to penicillin G. Human experience with the penicillins during pregnancy has not shown any positive evidence of adverse effects on the fetus. There are, however, no adequate and well controlled studies in pregnant women showing conclusively that harmful effects of these drugs on the fetus can be excluded. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Penicillins are excreted in human milk. Caution should be exercised when penicillins are administered to a nursing woman.
Pediatric Use
Incompletely developed renal function in newborns may delay elimination of penicillin; therefore, appropriate reductions in the dosage and frequency of administration should be made in these patients. All newborns treated with penicillins should be monitored closely for clinical and laboratory evidence of toxic or adverse effects (see Precautions).
Pediatric doses are generally determined on a weight basis and should be calculated for each patient individually. Recommended guidelines for pediatric dosages are presented in Dosage and Administration.
Geriatric Use
Clinical studies of penicillin G Injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Penicillin G Potassium for Injection, USP contains 6.8 mg (0.3 mEq) of sodium per million units. At the usual recommended doses of 10 to 20 million units per day patients would receive between 68 and 136 mg/day (3 and 6 mEq) of sodium per day. The geriatric population may respond with a blunted natriuresis to salt loading. This may be clinically important with regard to such diseases as congestive heart failure.
-
ADVERSE REACTIONS
Body as a whole
The Jarisch-Herxheimer reaction is a systemic reaction, that may occur after the initiation of penicillin therapy in patients with syphilis or other spirochetal infections (i.e., Lyme disease and Relapsing fever). The reaction begins one or two hours after initiation of therapy and disappears within 12 to 24 hours. It is characterized by fever, chills, myalgias, headache, exacerbation of cutaneous lesions, tachycardia, hyperventilation, vasodilation with flushing and mild hypotension. The pathogenesis of the Herxheimer reaction may be due to the release from the spirochetes of heat-stable pyrogen.
Hypersensitivity reactions
The reported incidence of allergic reactions to all penicillins ranges from 0.7 to 10 percent in different studies (see Warnings). Sensitization is usually the result of previous treatment with a penicillin, but some individuals have had immediate reactions when first treated. In such cases, it is postulated that prior exposure to penicillin may have occurred via trace amounts present in milk or vaccines.
Two types of allergic reactions to penicillin are noted clinically – immediate and delayed.
Immediate reactions usually occur within 20 minutes of administration and range in severity from urticaria and pruritus to angioneurotic edema, laryngospasm, bronchospasm, hypotension, vascular collapse and death (see Warnings). Such immediate anaphylactic reactions are very rare and usually occur after parenteral therapy, but a few cases of anaphylaxis have been reported following oral therapy. Another type of immediate reaction, an accelerated reaction, may occur between 20 minutes and 48 hours after administration and may include urticaria, pruritus, fever and, occasionally, laryngeal edema.
Delayed reactions to penicillin therapy usually occur within 1–2 weeks after initiation of therapy. Manifestations include serum sickness-like symptoms, i.e., fever, malaise, urticaria, myalgia, arthralgia, abdominal pain and various skin rashes, ranging from maculopapular eruptions to exfoliative dermatitis.
Contact dermatitis has been observed in individuals who prepare penicillin solutions.
Gastrointestinal system
Pseudomembranous colitis has been reported with the onset occurring during or after penicillin G treatment. Nausea, vomiting, stomatitis, black or hairy tongue, and other symptoms of gastrointestinal irritation may occur, especially during oral therapy.
Hematologic system
Reactions include neutropenia, which resolves after penicillin therapy is discontinued; Coombs-positive hemolytic anemia, an uncommon reaction, occurs in patients treated with intravenous penicillin G in doses greater than 10 million units/day and who have previously received large doses of the drug; and with large doses of penicillin, a bleeding diathesis can occur secondary to platelet dysfunction.
Metabolic
Penicillin G Potassium for Injection, USP (1 million units contains 1.68 mEq of potassium ion) may cause serious and even fatal electrolyte disturbances, i.e., hyperkalemia, when given intravenously in large doses.
Nervous system
Neurotoxic reactions including hyperreflexia, myoclonic twitches, seizures and coma have been reported following the administration of massive intravenous doses, and are more likely in patients with impaired renal function.
Urogenital system
Renal tubular damage and interstitial nephritis have been associated with large intravenous doses of penicillin G. Manifestations of this reaction may include fever, rash, eosinophilia, proteinuria, eosinophiluria, hematuria and a rise in serum urea nitrogen.
Discontinuation of penicillin G results in resolution in the majority of patients.
Local reactions
Phlebitis and thrombophlebitis may occur, and pain at the injection site has been reported with intravenous administration.
To report SUSPECTED ADVERSE EVENTS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or http://www.fda.gov/ for voluntary reporting of adverse reactions.
-
OVERDOSAGE
Dose related toxicity may arise with the use of massive doses of intravenous penicillins (40 to 100 million units per day), particularly in patients with severe renal impairment (see Precautions). The manifestations may include agitation, confusion, asterixis, hallucinations, stupor, coma, multifocal myoclonus, seizures and encephalopathy. Hyperkalemia is also possible (see Adverse Reactions-Metabolic).
In case of overdosage, discontinue penicillin, treat symptomatically and institute supportive measures as required. If necessary, hemodialysis may be used to reduce blood levels of Penicillin G, although the degree of effectiveness of this procedure is questionable.
-
DOSAGE AND ADMINISTRATION
Buffered Penicillin G Potassium for Injection, USP may be given intravenously or intramuscularly. The usual dose recommendations are as follows:
Adult patients
CLINICAL INDICATION DOSAGE - *
- Because of its short half-life, Penicillin G is administered in divided doses, usually every 4–6 hours with the exception of meningococcal meningitis/septicemia, i.e., every 2 hours.
Serious infections due to susceptible strains of streptococci (including S. pneumoniae)
-septicemia, empyema, pneumonia, pericarditis, endocarditis and meningitis12 to 24 million units/day depending on the infection and its severity administered in equally divided doses every 4–6 hours.
Serious infections due to susceptible strains of staphylococci
-septicemia, empyema, pneumonia, pericarditis, endocarditis and meningitis5 to 24 million units/day depending on the infection and its severity administered in equally divided doses every 4–6 hours.
Anthrax
Minimum of 8 million units/day in divided doses every 6 hours. Higher doses may be required depending on susceptibility of organism.
Actinomycosis
Cervicofacial disease
1 to 6 million units/day*
Thoracic and abdominal disease
10 to 20 million units/day*
Clostridial infections
Botulism (adjunctive therapy to antitoxin) Gas gangrene (debridement and/or surgery as indicated)
Tetanus (adjunctive therapy to human tetanus immune globulin)20 million units/day*
Diphtheria (adjunctive therapy to antitoxin and for the prevention of the carrier state)
2 to 3 million units/day in divided doses for 10–12 days*
Erysipelothrix endocarditis
12 to 20 million units/day for 4–6 weeks*
Fusospirochetosis (severe infections of the oropharynx [Vincent's], lower respiratory tract and genital area)
5 to 10 million units/day*
Listeria infections
Meningitis
15 to 20 million units/day for 2 weeks*
Endocarditis
15 to 20 million units/day for 4 weeks*
Pasteurella infections including bacteremia and meningitis
4 to 6 million units/day for 2 weeks*
Haverhill fever; Rat-bite fever
12 to 20 million units/day for 3–4 weeks*
Disseminated gonococcal infections, such as meningitis, endocarditis, arthritis, etc., caused by penicillin – susceptible organisms
10 million units/day*; duration depends on the type of infection
Syphilis (neurosyphilis)
12 to 24 million units/day, as 2–4 MU every 4 hours for 10–14 days; many experts recommend additional therapy with Benzathine PCN G 2.4 MU IM weekly for 3 doses after completion of IV therapy
Meningococcal meningitis and/or septicemia
24 million units/day as 2 million units every 2 hours
Pediatric patients
This product should not be administered to patients requiring less than one million units per dose (see Precautions-Pediatric Use).
CLINICAL INDICATION DOSAGE Serious infections, such as pneumonia and endocarditis, due to susceptible strains of streptococci (including S. pneumoniae) and meningococcus
150,000–300,000 units/kg/day divided in equal doses every 4–6 hours; duration depends on infecting organism and type of infection
Meningitis caused by susceptible strains of pneumococcus and meningococcus
250,000 units/kg/day divided in equal doses every 4 hours for 7–14 days depending on the infecting organism (maximum dose of 12–20 million units/day)
Disseminated Gonococcal Infections
(penicillin-susceptible strains)Weight less than 45 kg:
Arthritis
100,000 units/kg/day in 4 equally divided doses for 7–10 days
Meningitis
250,000 units/kg/day in equal doses every 4 hours for 10–14 days
Endocarditis
250,000 units/kg/day in equal doses every 4 hours for 4 weeks
Arthritis, meningitis, endocarditis
Weight 45 kg or greater: 10 million units/day in equally divided doses with the duration of therapy depending on the type of infection
Syphilis (congenital and neurosyphilis) after the newborn period
200,000–300,000 units/kg/day (administered as 50,000 units/kg every 4–6 hours) for 10–14 days
Diphtheria (adjunctive therapy to antitoxin and for prevention of the carrier state)
150,000–250,000 units/kg/day in equal doses every 6 hours for 7–10 days
Rat-bite fever; Haverhill fever (with endocarditis caused by S. moniliformis)
150,000–250,000 units/kg/day in equal doses every 4 hours for 4 weeks
Renal Impairment
Penicillin G is relatively nontoxic, and dosage adjustments are generally required only in cases of severe renal impairment. The recommended dosage regimens are as follows:
Creatinine clearance less than 10 mL/min/1.73m2; administer a full loading dose (see recommended dosages in the tables above) followed by one-half of the loading dose every 8–10 hours.
Uremic patients with a creatinine clearance greater than 10 mL/min/1.73m2; administer a full loading dose (see recommended dosages in the tables above) followed by one-half of the loading dose every 4–5 hours. Additional dosage modification should be made in patients with hepatic disease and renal impairment.
For most acute infections, treatment should be continued for at least 48 to 72 hours after the patient becomes asymptomatic. Antibiotic therapy for Group A β-hemolytic streptococcal infections should be maintained for at least 10 days to reduce the risk of rheumatic fever.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Reconstitution
The following table shows the amount of solvent required for solution of various concentrations:
Approx. Desired Concentration
(units/mL)Volume (mL)
Solvent for Vial of 5,000,000 unitsInfusion Only
Volume (mL)
Solvent for Vial of 20,000,000 units50,000
–
–
100,000
–
–
250,000
18.2
75.0
500,000
8.2
33.0
1,000,000
3.2
11.5
When the required volume of solvent is greater than the capacity of the vial, the penicillin can be dissolved by first injecting only a portion of the solvent into the vial, then withdrawing the resultant solution and combining it with the remainder of the solvent in a larger sterile container.
Buffered Pfizerpen (Penicillin G Potassium for Injection, USP) is highly water soluble. It may be dissolved in small amounts of Water for Injection, or Sterile Isotonic Sodium Chloride Solution for Parenteral Use. All solutions should be stored in a refrigerator. When refrigerated, penicillin solutions may be stored for seven days without significant loss of potency.
Buffered Pfizerpen for Injection may be given intramuscularly or by continuous intravenous infusion for dosages of 500,000, 1,000,000, or 5,000,000 units. It is also suitable for intrapleural, intraarticular, and other local instillations.
THE 20,000,000 UNIT DOSAGE MAY BE ADMINISTERED BY INTRAVENOUS INFUSION ONLY.
(1) Intramuscular Injection
Keep total volume of injection small. The intramuscular route is the preferred route of administration. Solutions containing up to 100,000 units of penicillin per mL of diluent may be used with a minimum of discomfort. Greater concentration of penicillin G per mL is physically possible and may be employed where therapy demands. When large dosages are required, it may be advisable to administer aqueous solutions of penicillin by means of continuous intravenous infusion.
(2) Continuous Intravenous Infusion
Determine the volume of fluid and rate of its administration required by the patient in a 24 hour period in the usual manner for fluid therapy, and add the appropriate daily dosage of penicillin to this fluid. For example, if an adult patient requires 2 liters of fluid in 24 hours and a daily dosage of 10 million units of penicillin, add 5 million units to 1 liter and adjust the rate of flow so the liter will be infused in 12 hours.
(3) Intrapleural or Other Local Infusion
If fluid is aspirated, give infusion in a volume equal to ¼ or ½ the amount of fluid aspirated, otherwise, prepare as for intramuscular injection.
(4) Intrathecal Use
The intrathecal use of penicillin in meningitis must be highly individualized. It should be employed only with full consideration of the possible irritating effects of penicillin when used by this route. The preferred route of therapy in bacterial meningitides is intravenous, supplemented by intramuscular injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Sterile solution may be left in refrigerator for one week without significant loss of potency.
-
HOW SUPPLIED
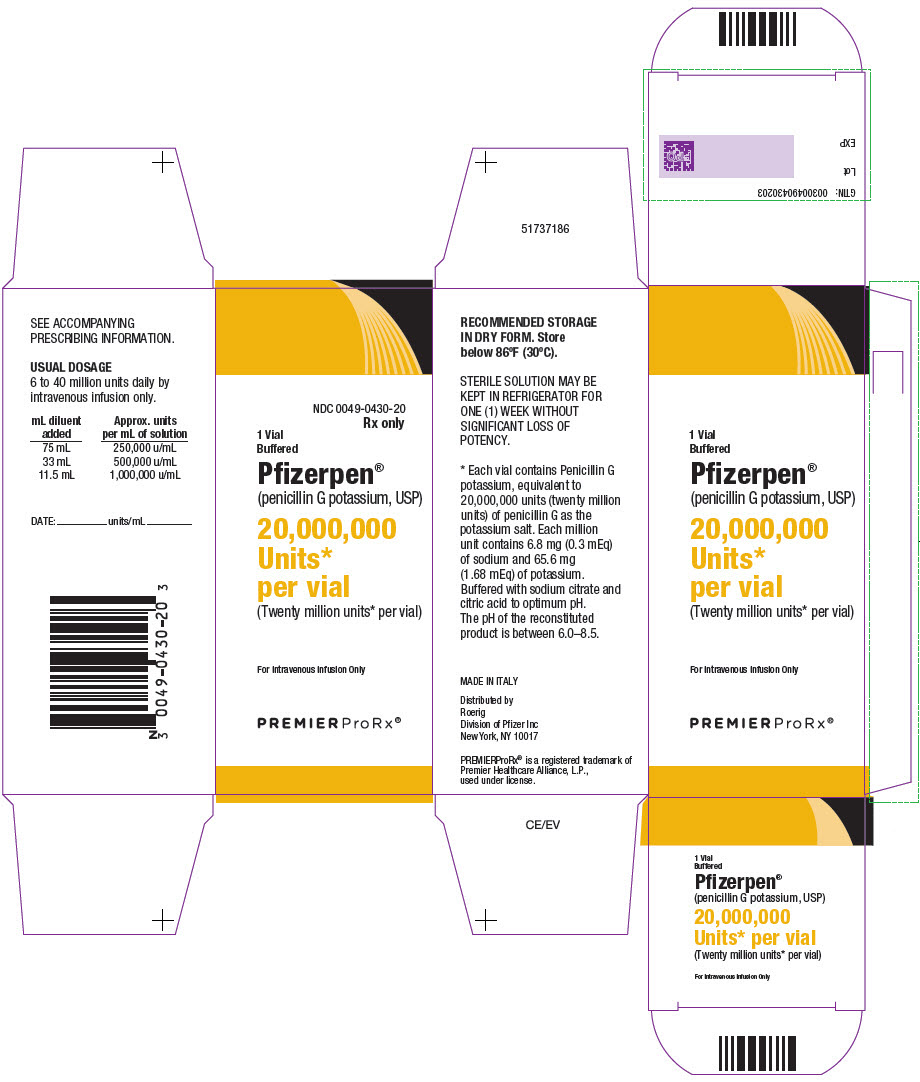
Buffered Pfizerpen® (Penicillin G Potassium for Injection, USP) is available in the following package sizes, buffered with sodium citrate and citric acid to an optimum pH:
10 vials per carton
5,000,000 units each vial
NDC 0049-0420-10
(each 1 vial contains 5,000,000 units, NDC 0049-0420-05)
1 vial per carton
20,000,000 units
NDC 0049-0430-20
Each million units contains approximately 6.8 milligrams of sodium (0.3 mEq) and 65.6 milligrams of potassium (1.68 mEq).
-
REFERENCES
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
For medical information about Buffered Pfizerpen, please visit www.pfizermedinfo.com or call 1‑800‑438‑1985.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 5,000,000 Unit Vial Label
- PRINCIPAL DISPLAY PANEL - 5,000,000 Unit Vial Carton
- PRINCIPAL DISPLAY PANEL - 20,000,000 Unit Vial Label
- PRINCIPAL DISPLAY PANEL - 20,000,000 Unit Vial Carton
-
INGREDIENTS AND APPEARANCE
PFIZERPEN
penicillin g potassium powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0049-0420 Route of Administration INTRAVENOUS, INTRAMUSCULAR, INTRAPLEURAL, INTRATHECAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PENICILLIN G POTASSIUM (UNII: VL775ZTH4C) (PENICILLIN G - UNII:Q42T66VG0C) PENICILLIN G 5000000 [iU] Inactive Ingredients Ingredient Name Strength SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0049-0420-10 10 in 1 CARTON 03/30/2018 1 NDC:0049-0420-05 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA060657 06/01/2010 PFIZERPEN
penicillin g potassium powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0049-0430 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PENICILLIN G POTASSIUM (UNII: VL775ZTH4C) (PENICILLIN G - UNII:Q42T66VG0C) PENICILLIN G 20000000 [iU] Inactive Ingredients Ingredient Name Strength SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0049-0430-20 1 in 1 CARTON 03/30/2018 1 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA060657 06/01/2010 Labeler - Roerig (829076996) Establishment Name Address ID/FEI Business Operations Pharmacia & Upjohn Company LLC 618054084 ANALYSIS(0049-0420, 0049-0430)