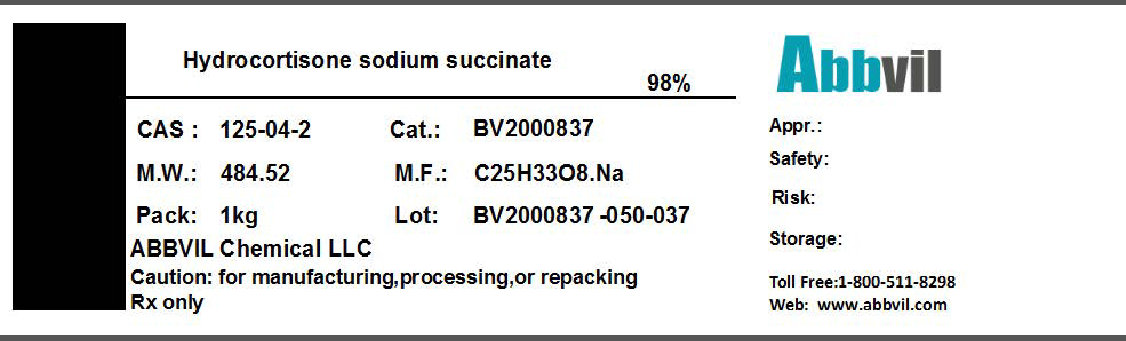
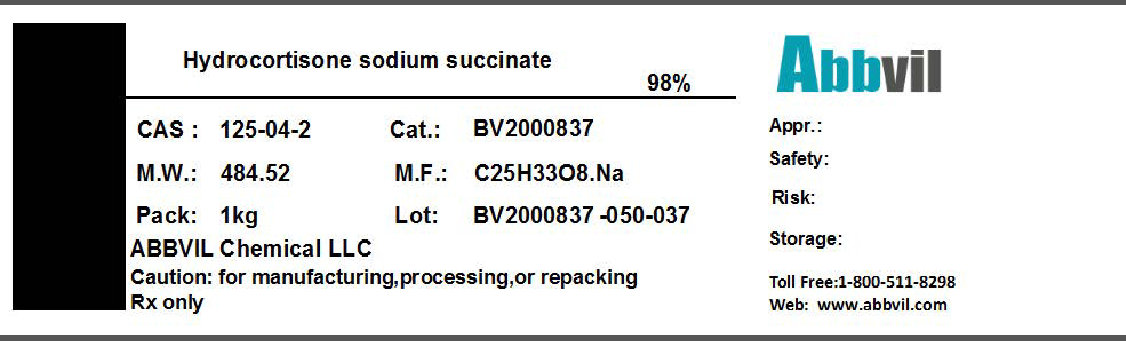
Label: HYDROCORTISONE SODIUM SUCCINATE powder
- NDC Code(s): 86065-001-01
- Packager: Abbvil Chemical LLC
- Category: BULK INGREDIENT
- DEA Schedule: None
- Marketing Status: Bulk Ingredient For Animal Drug Compounding
Drug Label Information
Updated April 8, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Hydrocortisone Sodium Succinate
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE SODIUM SUCCINATE
hydrocortisone sodium succinate powderProduct Information Product Type BULK INGREDIENT Item Code (Source) NDC:86065-001 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE SODIUM SUCCINATE (UNII: 50LQB69S1Z) (HYDROCORTISONE SODIUM SUCCINATE - UNII:50LQB69S1Z) HYDROCORTISONE SODIUM SUCCINATE 0.98 kg in 1 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86065-001-01 1 kg in 1 CONTAINER 04/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BULK INGREDIENT FOR ANIMAL DRUG COMPOUNDING 04/01/2016 Labeler - Abbvil Chemical LLC (005448211) Establishment Name Address ID/FEI Business Operations Abbvil Chemical LLC 005448211 relabel(86065-001) Establishment Name Address ID/FEI Business Operations Henan Lihua Pharmaceutical Co Ltd 421276878 api manufacture(86065-001)