Label: SILATRIX ORAL- sucralfate gel
- NHRIC Code(s): 69420-8351-1
- Packager: SA3, LLC
- Category: MEDICAL DEVICE
- DEA Schedule: None
- Marketing Status: Premarket Notification
Drug Label Information
Updated January 24, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
SILATRIX ORAL GEL is an amorphous hydrogel formed by the controlled reaction of sucralfate with a limited quantity of malic acid and calcium carbonate solution. The amorphous hydrogel formed by this reaction binds reversibly to wounds and is intended to form a protective film that covers wounds, protects against further irritation and relieves pain.
Silatrix Oral Gel may be administered directly to an accessible oral wound to provide an adherent physical covering of the wound bed. Although prepared by reaction of sucralfate with an acid, the polymerized sucralfate self-buffers to a pH 5.0 - 7 .0.
- INGREDIENTS
-
INDICATIONS AND USES
SILATRIX ORAL GEL forms a protective layer over the oral mucosa by adhering to the mucosal surface which allows it to protect against further irritation and relieve pain. The oral gel may be used in the management of mouth lesions including aphthous ulcer, stomatitis, mucositis, minor lesions, chafing and traumatic ulcers, abrasions caused by braces and ill-fitting dentures, and lesions associated with oral surgery. - CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Stop use and ask a doctor if irritation develops. SILATRIX ORAL GEL has no known serious side effects or adverse reactions. This medication should be used as directed by your physician during pregnancy or while breastfeeding. Consult your doctor about the risks and benefits.
CAUTION: Federal law restricts this device to sale by or on the order of a physician or other practitioner licensed by the law of the State in which the practitioner practices to use or order the use of the device.
- DOSAGE AND ADMINISTRATION
- HOW SILATRIX ORAL GEL IS SUPPLIED
- STORAGE:
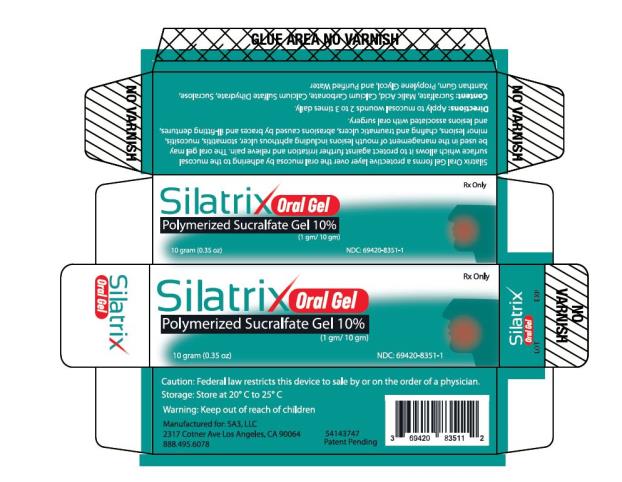
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SILATRIX ORAL
oral wound dressing gelProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:69420-8351 Route of Administration ORAL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:69420-8351-1 10 in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Premarket Notification K202000 02/17/2021 Labeler - SA3, LLC (079627454)