Label: NEOMULTIVITE tablet
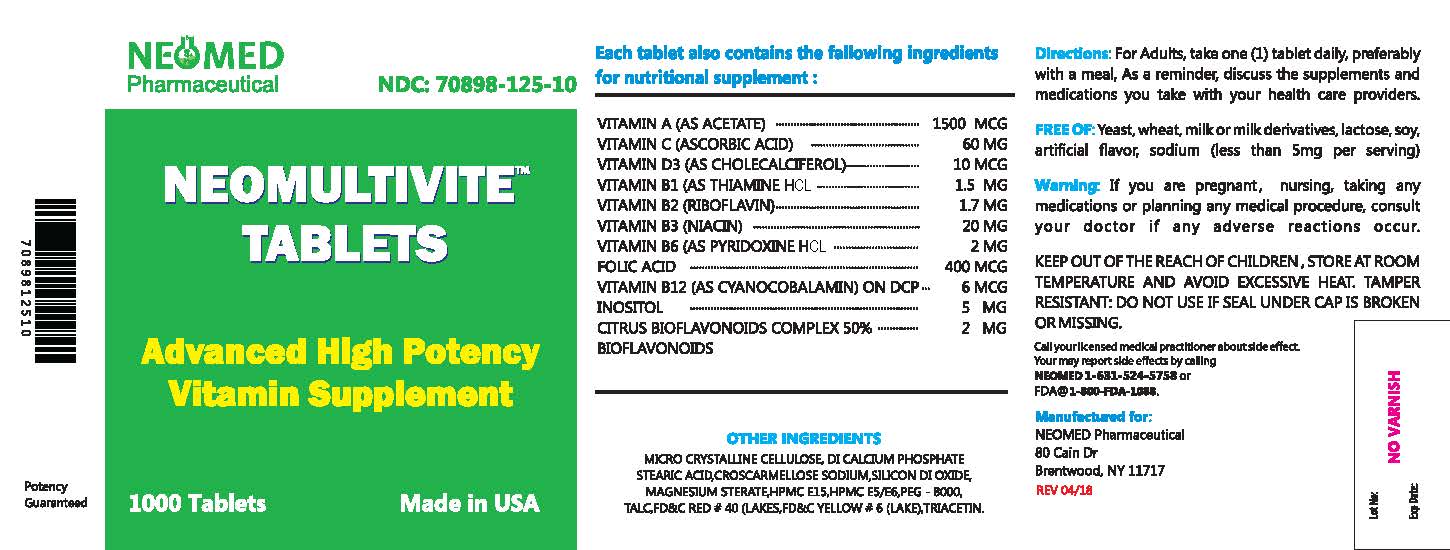
- NHRIC Code(s): 70898-125-10
- Packager: NEOMED PHARMACEUTICAL
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated September 13, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
SUPPLEMENT FACTS:
Serving Size: One Tablet
Each Tablet contains:
Vitamin A (as acetate)
1500 mcg
Vitamin D3 (as cholecalciferol)
10 mcg
Vitamin C (Ascorbic acid )
60 mcg
Vitamin B1 (as thiamine HCl)
1.5 mg
Vitamin B2 (as riboflavin)
1.7 mg
Niacin (Vitamin B3)
20 mg
Vitamin B6 (as pyridoxine HCl)
2 mg
Folic acid
400 mcg
Vitamin B12 (as cyanocobalamin)
6 mcg
Citrus bioflavonoids complex 50% bio flavonoids
2 mg
Inositol
5 mg
- Other Ingredients: Di calcium phosphate, microcrystalline croscarmellose sodium, stearic acid, silicon dioxide, titanium dioxide (as color), polyethylene glycol, magnesium stearate, talc,triacetin, HPMC E15,HPMC E5/E6,FD&C RED #40 (LAKES),FD&C YELLOW # 6 (LAKE )
-
WARNINGS
CONTRAINDICATIONS
NEOMULTIVITE TABLETS contraindicated in patients with hypersensitivity to any of its components. Folic Acid is contraindicated in patients with untreated and uncomplicated pernicious anemia, and in those with anaphylactic sensitivity to folic acid.
Cyanocobalamin is contraindicated in patients with sensitivity to cobalt or to cyanocobalamin (Vitamin B12)
WARNING/PRECAUTIONS
Vitamin D supplementation should be used with caution in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones. High doses of vitamin D can lead to elevated levels of calcium that reside in the blood and soft tissues. Bone pain, high blood pressure, formation of kidney stones, renal failure, and increased risk of heart disease can occur.
Avoid Over dosage. Keep out of the reach of children.
Information for Patients
Patients should be counseled to disclose all medical conditions, including use of all medications, vitamins and supplements, pregnancy, and breastfeeding.
Pediatric Use
Not recommended for pediatric use.
-
HEALTH CLAIM
DOSAGE AND ADMINISTRATION
Adults - Take one tablet daily with water, as a dietary supplement or as directed by a doctor.
HOW SUPPLIED
NEOMULTIVITE ; available in bottles of 1000 Tablets. 70898-125-10
Store at room temperature 15°-30°C (59°-86°F). Avoid excessive heat and moisture.
Distributed by:
Neomed Pharmaceutical
80 Cain Dr, Brentwood, NY 11717
Phone :631-524-5758Email: info@neomedpharmaceutical.com
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
- undefined
-
INGREDIENTS AND APPEARANCE
NEOMULTIVITE
neomultivite tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:70898-125 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A (UNII: 81G40H8B0T) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 1500 ug ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 60 ug CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 10 ug THIAMINE (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.5 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1.7 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 2 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 400 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 6 ug CITRUS BIOFLAVONOIDS (UNII: BD70459I50) (HESPERIDIN - UNII:E750O06Y6O) CITRUS BIOFLAVONOIDS 2 mg INOSITOL (UNII: 4L6452S749) (INOSITOL - UNII:4L6452S749) INOSITOL 5 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) HYPROMELLOSES (UNII: 3NXW29V3WO) TRIACETIN (UNII: XHX3C3X673) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:70898-125-10 1 in 1 PACKAGE 1 1000 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 07/31/2018 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 7 mm Labeler - NEOMED PHARMACEUTICAL (048388130)