Label: ENGERIX-B (hepatitis b vaccine- recombinant injection, suspension
-
NDC Code(s):
58160-820-43,
58160-820-52,
58160-821-01,
58160-821-05, view more58160-821-11, 58160-821-34, 58160-821-43, 58160-821-52
- Packager: GlaxoSmithKline Biologicals SA
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ENGERIX-B safely and effectively. See full prescribing information for ENGERIX-B.
ENGERIX-B [Hepatitis B Vaccine (Recombinant)] injectable suspension, for intramuscular use
Initial U.S. Approval: 1989RECENT MAJOR CHANGES
- Warnings and Precautions, Latex (5.1) - Removed
- 10/2023
INDICATIONS AND USAGE
ENGERIX-B is a vaccine indicated for immunization against infection caused by all known subtypes of hepatitis B virus. (1)
DOSAGE AND ADMINISTRATION
- •
- Persons from birth through 19 years of age: A series of 3 doses (0.5 mL each) on a 0-, 1-, 6-month schedule. (2.3)
- •
- Persons 20 years of age and older: A series of 3 doses (1 mL each) on a 0-, 1-, 6-month schedule. (2.3)
- •
- Adults on hemodialysis: A series of 4 doses (2 mL each) as a single 2-mL dose or as two 1-mL doses on a 0-, 1-, 2-, 6-month schedule. (2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) after a previous dose of any hepatitis B-containing vaccine, or to any component of ENGERIX-B, including yeast. (4)
WARNINGS AND PRECAUTIONS
- •
- Syncope (fainting) can occur in association with administration of injectable vaccines, including ENGERIX-B. Procedures should be in place to avoid falling injury and to restore cerebral perfusion following syncope. (5.1)
- •
- Temporarily defer vaccination of infants with a birth weight less than 2,000 g born to hepatitis B surface antigen (HBsAg)-negative mothers. (5.2)
- •
- Apnea following intramuscular vaccination has been observed in some infants born prematurely. Decisions about when to administer an intramuscular vaccine, including ENGERIX-B, to infants born prematurely should be based on consideration of the infant’s medical status, and the potential benefits and possible risks of vaccination. (5.3)
ADVERSE REACTIONS
The most common solicited adverse reactions were injection-site soreness (22%) and fatigue (14%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
DRUG INTERACTIONS
Do not mix ENGERIX-B with any other vaccine or product in the same syringe or vial. (7.1)
USE IN SPECIFIC POPULATIONS
Antibody responses are lower in persons older than 60 years than in younger adults. (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Preparation for Administration
2.2 Administration
2.3 Recommended Dose and Schedule
2.4 Alternate Dosing Schedules
2.5 Booster Vaccinations
2.6 Known or Presumed Exposure to Hepatitis B Virus
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Syncope
5.2 Infants Weighing Less than 2,000 g at Birth
5.3 Apnea in Premature Infants
5.4 Preventing and Managing Allergic Vaccine Reactions
5.5 Moderate or Severe Acute Illness
5.6 Altered Immunocompetence
5.7 Multiple Sclerosis
5.8 Limitations of Vaccine Effectiveness
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Vaccines and Immune Globulin
7.2 Interference with Laboratory Tests
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Efficacy in Neonates
14.2 Efficacy and Immunogenicity in Specific Populations
14.3 Immunogenicity in Neonates
14.4 Immunogenicity in Children and Adults
14.5 Interchangeability with Other Hepatitis B Vaccines
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For intramuscular administration. See Section 2.2 for subcutaneous administration in persons at risk of hemorrhage.
2.1 Preparation for Administration
Shake well before use. With thorough agitation, ENGERIX-B is a homogeneous, turbid white suspension. Do not administer if it appears otherwise. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered.
For the prefilled syringes, attach a sterile needle and administer intramuscularly.
For the vials, use a sterile needle and sterile syringe to withdraw the vaccine dose and administer intramuscularly. Changing needles between drawing vaccine from a vial and injecting it into a recipient is not necessary unless the needle has been damaged or contaminated. Use a separate sterile needle and syringe for each individual.
2.2 Administration
ENGERIX-B should be administered by intramuscular injection. The preferred administration site is the anterolateral aspect of the thigh for infants younger than 1 year and the deltoid muscle in older children (whose deltoid is large enough for an intramuscular injection) and adults. ENGERIX-B should not be administered in the gluteal region; such injections may result in suboptimal response.
ENGERIX-B may be administered subcutaneously to persons at risk of hemorrhage (e.g., hemophiliacs). However, hepatitis B vaccines administered subcutaneously are known to result in a lower antibody response. Additionally, when other aluminum-adsorbed vaccines have been administered subcutaneously, an increased incidence of local reactions including subcutaneous nodules has been observed. Therefore, subcutaneous administration should be used only in persons who are at risk of hemorrhage with intramuscular injections.
Do not administer this product intravenously or intradermally.
2.3 Recommended Dose and Schedule
Persons from Birth through 19 Years
Primary immunization for infants (born of hepatitis B surface antigen [HBsAg]-negative or HBsAg-positive mothers), children (birth through 10 years), and adolescents (aged 11 through 19 years) consists of a series of 3 doses (0.5 mL each) given on a 0-, 1-, and 6-month schedule.
Persons Aged 20 Years and Older
Primary immunization for persons aged 20 years and older consists of a series of 3 doses (1 mL each) given on a 0-, 1-, and 6-month schedule.
Adults on Hemodialysis
Primary immunization consists of a series of 4 doses (2-mL each) given as a single 2-mL dose or two 1-mL doses on a 0-, 1-, 2-, and 6-month schedule. In hemodialysis patients, antibody response is lower than in healthy persons and protection may persist only as long as antibody levels remain above 10 mIU/mL. Therefore, the need for booster doses should be assessed by annual antibody testing. A 2-mL booster dose (as a single 2-mL dose or two 1-mL doses) should be given when antibody levels decline below 10 mIU/mL.1 [See Clinical Studies (14.2).]
Table 1. Recommended Dosage and Administration Schedules Group
Dosea
Schedules
Infants born of:
HBsAg-negative mothers
0.5 mL
0, 1, 6 months
HBsAg-positive mothersb
0.5 mL
0, 1, 6 months
Children:
Birth through 10 years
0.5 mL
0, 1, 6 months
Adolescents:
Aged 11 through 19 years
0.5 mL
0, 1, 6 months
Adults:
Aged 20 years and older
1 mL
0, 1, 6 months
Adults on hemodialysis
2 mLc
0, 1, 2, 6 months
HBsAg = Hepatitis B surface antigen.
a 0.5 mL (10 mcg); 1 mL (20 mcg).
b Infants born to HBsAg-positive mothers should receive vaccine and hepatitis B immune globulin (HBIG) within 12 hours after birth [see Dosage and Administration (2.6)].
c Given as a single 2-mL dose or as two 1-mL doses.
2.4 Alternate Dosing Schedules
There are alternate dosing and administration schedules which may be used for specific populations (e.g., neonates born of hepatitis B–infected mothers, persons who have or might have been recently exposed to the virus, and travelers to high-risk areas) (Table 2). For some of these alternate schedules, an additional dose at 12 months is recommended for prolonged maintenance of protective titers.
Table 2. Alternate Dosage and Administration Schedules Group
Dosea
Schedules
Infants born of:
HBsAg-positive mothersb
0.5 mL
0, 1, 2, 12 months
Children:
Birth through 10 years
0.5 mL
0, 1, 2, 12 months
Aged 5 through 10 years
0.5 mL
0, 12, 24 monthsc
Adolescents:
Aged 11 through 16 years
0.5 mL
0, 12, 24 monthsc
Aged 11 through 19 years
1 mL
0, 1, 6 months
Aged 11 through 19 years
1 mL
0, 1, 2, 12 months
Adults:
Aged 20 years and older
1 mL
0, 1, 2, 12 months
HBsAg = Hepatitis B surface antigen.
a 0.5 mL (10 mcg); 1 mL (20 mcg).
b Infants born to HBsAg-positive mothers should receive vaccine and hepatitis B immune globulin (HBIG) within 12 hours after birth [see Dosage and Administration (2.6)].
c For children and adolescents for whom an extended administration schedule is acceptable based on risk of exposure.
2.5 Booster Vaccinations
Whenever administration of a booster dose is appropriate, the dose of ENGERIX-B is 0.5 mL for children aged 10 years and younger and 1 mL for persons aged 11 years and older. Studies have demonstrated a substantial increase in antibody titers after booster vaccination with ENGERIX-B. See Section 2.3 for information on booster vaccination for adults on hemodialysis.
2.6 Known or Presumed Exposure to Hepatitis B Virus
Persons with known or presumed exposure to the hepatitis B virus (e.g., neonates born of infected mothers, persons who experienced percutaneous or permucosal exposure to the virus) should be given hepatitis B immune globulin (HBIG) in addition to ENGERIX-B in accordance with Advisory Committee on Immunization Practices recommendations and with the package insert for HBIG. ENGERIX-B can be given on either dosing schedule (0, 1, and 6 months or 0, 1, 2, and 12 months).
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) after a previous dose of any hepatitis B-containing vaccine, or to any component of ENGERIX-B, including yeast, is a contraindication to administration of ENGERIX-B [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Syncope
Syncope (fainting) can occur in association with administration of injectable vaccines, including ENGERIX-B. Syncope can be accompanied by transient neurological signs such as visual disturbance, paresthesia, and tonic-clonic limb movements. Procedures should be in place to avoid falling injury and to restore cerebral perfusion following syncope.
5.2 Infants Weighing Less than 2,000 g at Birth
Hepatitis B vaccine should be deferred for infants with a birth weight <2,000 g if the mother is documented to be HBsAg negative at the time of the infant’s birth. Vaccination can commence at chronological age 1 month or hospital discharge. Infants born weighing <2,000 g to HBsAg-positive mothers should receive vaccine and HBIG within 12 hours after birth. Infants born weighing <2,000 g to mothers of unknown HBsAg status should receive vaccine and HBIG within 12 hours after birth if the mother’s HBsAg status cannot be determined within the first 12 hours of life. The birth dose in infants born weighing <2,000 g should not be counted as the first dose in the vaccine series and it should be followed with a full 3-dose standard regimen (total of 4 doses).2 [See Dosage and Administration (2).]
5.3 Apnea in Premature Infants
Apnea following intramuscular vaccination has been observed in some infants born prematurely. Decisions about when to administer an intramuscular vaccine, including ENGERIX-B, to infants born prematurely should be based on consideration of the infant’s medical status, and the potential benefits and possible risks of vaccination. For ENGERIX-B, this assessment should include consideration of the mother’s hepatitis B antigen status and the high probability of maternal transmission of hepatitis B virus to infants born of mothers who are HBsAg positive if vaccination is delayed.
5.4 Preventing and Managing Allergic Vaccine Reactions
Prior to immunization, the healthcare provider should review the immunization history for possible vaccine sensitivity and previous vaccination-related adverse reactions to allow an assessment of benefits and risks. Epinephrine and other appropriate agents used for the control of immediate allergic reactions must be immediately available should an acute anaphylactic reaction occur. [See Contraindications (4).]
5.5 Moderate or Severe Acute Illness
To avoid diagnostic confusion between manifestations of an acute illness and possible vaccine adverse effects, vaccination with ENGERIX-B should be postponed in persons with moderate or severe acute febrile illness unless they are at immediate risk of hepatitis B infection (e.g., infants born of HBsAg-positive mothers).
5.6 Altered Immunocompetence
Immunocompromised persons may have a diminished immune response to ENGERIX-B, including individuals receiving immunosuppressant therapy.
5.7 Multiple Sclerosis
Results from 2 clinical studies indicate that there is no association between hepatitis B vaccination and the development of multiple sclerosis,3 and that vaccination with hepatitis B vaccine does not appear to increase the short‑term risk of relapse in multiple sclerosis.4
5.8 Limitations of Vaccine Effectiveness
Hepatitis B has a long incubation period. ENGERIX-B may not prevent hepatitis B infection in individuals who had an unrecognized hepatitis B infection at the time of vaccine administration. Additionally, it may not prevent infection in individuals who do not achieve protective antibody titers.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The most common solicited adverse reactions were injection site soreness (22%) and fatigue (14%).
In 36 clinical studies, a total of 13,495 doses of ENGERIX-B were administered to 5,071 healthy adults and children who were initially seronegative for hepatitis B markers, and healthy neonates. All subjects were monitored for 4 days post-administration. Frequency of adverse reactions tended to decrease with successive doses of ENGERIX-B.
Using a symptom checklist, the most frequently reported adverse reactions were injection site soreness (22%) and fatigue (14%). Other reactions are listed below. Parent or guardian completed forms for children and neonates. Neonatal checklist did not include headache, fatigue, or dizziness.
Incidence 1% to 10% of Injections
Nervous System Disorders: Dizziness, headache.
General Disorders and Administration Site Conditions: Fever (>37.5°C), injection site erythema, injection site induration, injection site swelling.
Incidence <1% of Injections
Infections and Infestations: Upper respiratory tract illnesses.
Blood and Lymphatic System Disorders: Lymphadenopathy.
Metabolism and Nutrition Disorders: Anorexia.
Psychiatric Disorders: Agitation, insomnia.
Nervous System Disorders: Somnolence, tingling.
Vascular Disorders: Flushing, hypotension.
Gastrointestinal Disorders: Abdominal pain/cramps, constipation, diarrhea, nausea, vomiting.
Skin and Subcutaneous Tissue Disorders: Erythema, petechiae, pruritus, rash, sweating, urticaria.
Musculoskeletal and Connective Tissue Disorders: Arthralgia, back pain, myalgia, pain/stiffness in arm, shoulder, or neck.
General Disorders and Administration Site Conditions: Chills, influenza-like symptoms, injection site ecchymosis, injection site pain, injection site pruritus, irritability, malaise, weakness.
In a clinical trial, 416 adults with type 2 diabetes and 258 control subjects without type 2 diabetes who were seronegative for hepatitis B markers received at least 1 dose of ENGERIX-B. Subjects were monitored for solicited adverse reactions for 4 days following each vaccination. The most frequently reported solicited adverse reactions in the entire study population were injection site pain (reported in 39% of diabetic subjects and 45% of control subjects) and fatigue (reported in 29% of diabetic subjects and 27% of control subjects). Serious adverse events were monitored through 30 days following the last vaccination. Serious adverse events (SAEs) occurred in 3.8% of diabetic subjects and 1.6% of controls. No SAEs were deemed related to ENGERIX-B.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ENGERIX-B. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the vaccine.
Infections and Infestations
Herpes zoster, meningitis.
Blood and Lymphatic System Disorders
Thrombocytopenia.
Immune System Disorders
Allergic reaction, anaphylactoid reaction, anaphylaxis. An apparent hypersensitivity syndrome (serum sickness-like) of delayed onset has been reported days to weeks after vaccination, including: arthralgia/arthritis (usually transient), fever, and dermatologic reactions such as urticaria, erythema multiforme, ecchymoses, and erythema nodosum.
Nervous System Disorders
Encephalitis; encephalopathy; migraine; multiple sclerosis; neuritis; neuropathy including hypoesthesia, paresthesia, Guillain-Barré syndrome and Bell’s palsy; optic neuritis; paralysis; paresis; seizures; syncope; transverse myelitis.
Eye Disorders
Conjunctivitis, keratitis, visual disturbances.
Ear and Labyrinth Disorders
Earache, tinnitus, vertigo.
Cardiac Disorders
Palpitations, tachycardia.
Vascular Disorders
Vasculitis.
Respiratory, Thoracic, and Mediastinal Disorders
Apnea, bronchospasm including asthma-like symptoms.
Gastrointestinal Disorders
Dyspepsia.
Skin and Subcutaneous Tissue Disorders
Alopecia, angioedema, eczema, erythema multiforme including Stevens-Johnson syndrome, erythema nodosum, lichen planus, purpura.
Musculoskeletal and Connective Tissue Disorders
Arthritis, muscular weakness.
General Disorders and Administration Site Conditions
Injection site reaction.
Investigations
Abnormal liver function tests.
-
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Vaccines and Immune Globulin
ENGERIX-B may be administered concomitantly with immune globulin.
When concomitant administration of other vaccines or immune globulin is required, they should be given with different syringes and at different injection sites. Do not mix ENGERIX-B with any other vaccine or product in the same syringe or vial.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
There are no adequate and well-controlled studies of ENGERIX-B in pregnant women in the U.S. Available data do not suggest an increased risk of major birth defects and miscarriage in women who received ENGERIX-B during pregnancy (see Data).
There are no animal studies with ENGERIX-B to inform use during pregnancy. A developmental toxicity study was performed in female rats administered a vaccine with the same hepatitis B surface antigen component and quantity as ENGERIX-B prior to mating and during gestation (0.2 mL at each occasion). This study revealed no adverse effects on fetal or pre-weaning development (see Data).
Data
Human Data: In an evaluation of pre- and post-licensure clinical trials of ENGERIX-B, 58 pregnant women were inadvertently administered ENGERIX-B following their last menstrual period. After excluding elective terminations (n = 6), those with an unknown outcome (n = 3), those with exposure in the third trimester (n = 1), and those with an unknown exposure timing (n = 22), there were 26 pregnancies with known outcomes with exposure in the first or second trimester. Miscarriage was reported in 11.5% of pregnancies with exposure prior to 20 weeks of gestation (3/26) and major birth defects were reported in 0% (0/23) of live births born to women with exposure during the first or second trimester. The rates of miscarriage and major birth defects were consistent with estimated background rates.
No pregnancy registry for ENGERIX-B was conducted. TWINRIX [Hepatitis A & Hepatitis B (Recombinant) Vaccine] is a bivalent vaccine containing the same hepatitis B surface antigen component and quantity as used in ENGERIX-B. Therefore, clinical data accrued with TWINRIX are relevant to ENGERIX-B. A pregnancy exposure registry was maintained for TWINRIX from 2001 to 2015. The registry prospectively enrolled 245 women who received a dose of TWINRIX during pregnancy or within 28 days prior to conception. After excluding induced abortions (n = 6, including one of a fetus with congenital anomalies), those lost to follow-up (n = 142), those with exposure in the third trimester (n = 1), and those with an unknown exposure timing (n = 9), there were 87 pregnancies with known outcomes with exposure within 28 days prior to conception, or in the first or second trimesters. Miscarriage was reported for 9.6% of pregnancies with exposure to TWINRIX prior to 20 weeks gestation (8/83). Major birth defects were reported for 3.8% of live born infants whose mothers were exposed within 28 days prior to conception or during the first or second trimester (3/80). The rates of miscarriage and major birth defects were consistent with estimated background rates.
Animal Data: In a developmental toxicity study, female rats were administered TWINRIX, which contains the same hepatitis B surface antigen component and quantity as ENGERIX-B, by intramuscular injection on Day 30 prior to mating and on gestation Days 6, 8, 11, and 15. The total dose was 0.2 mL (divided) at each occasion (a single human dose is 1 mL). No adverse effects on pre-weaning development up to post-natal Day 25 were observed. There were no fetal malformations or variations.
8.2 Lactation
Risk Summary
There is no information regarding the presence of ENGERIX-B in human milk, the effects on the breastfed child, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ENGERIX-B and any potential adverse effects on the breastfed child from ENGERIX-B or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
Safety and effectiveness of ENGERIX-B have been established in all pediatric age-groups. Maternally transferred antibodies do not interfere with the active immune response to the vaccine. [See Adverse Reactions (6), Clinical Studies (14.1, 14.3, 14.4).]
The timing of the first dose in infants weighing less than 2,000 g at birth depends on the HBsAg status of the mother. [See Warnings and Precautions (5.2).]
8.5 Geriatric Use
Clinical studies of ENGERIX-B used for licensure did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently from younger subjects. However, in later studies it has been shown that a diminished antibody response and seroprotective levels can be expected in persons older than 60 years.5 [See Clinical Studies (14.2).]
-
11 DESCRIPTION
ENGERIX-B [Hepatitis B Vaccine (Recombinant)] is a sterile suspension of noninfectious HBsAg for intramuscular administration. It contains purified surface antigen of the virus obtained by culturing genetically engineered Saccharomyces cerevisiae cells, which carry the surface antigen gene of the hepatitis B virus. The HBsAg expressed in the cells is purified by several physicochemical steps and formulated as a suspension of the antigen adsorbed on aluminum hydroxide. The procedures used to manufacture ENGERIX-B result in a product that contains no more than 5% yeast protein.
Each 0.5-mL pediatric/adolescent dose contains 10 mcg of HBsAg adsorbed on 0.25 mg aluminum as aluminum hydroxide.
Each 1-mL adult dose contains 20 mcg of HBsAg adsorbed on 0.5 mg aluminum as aluminum hydroxide.
ENGERIX-B contains the following excipients: Sodium chloride (8 mg/mL) and phosphate buffers (disodium phosphate dihydrate, 0.9 mg/mL; sodium dihydrogen phosphate dihydrate, 0.7 mg/mL).
ENGERIX‑B is available in vials (adult dose only) and prefilled syringes. The tip cap and rubber plunger stopper of the prefilled syringe are not made with natural rubber latex. The vial stoppers are not made with natural rubber latex.
ENGERIX-B is formulated without preservatives.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Infection with hepatitis B virus can have serious consequences including acute massive hepatic necrosis and chronic active hepatitis. Chronically infected persons are at increased risk for cirrhosis and hepatocellular carcinoma.
Antibody concentrations ≥10 mIU/mL against HBsAg are recognized as conferring protection against hepatitis B virus infection.1 Seroconversion is defined as antibody titers ≥1 mIU/mL.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
ENGERIX‑B has not been evaluated for carcinogenic or mutagenic potential, or for impairment of male fertility in animals. Vaccination of female rats with TWINRIX, which contains the same HBsAg component and quantity as ENGERIX-B, had no effect on fertility. [See Use in Specific Populations (8.1).]
-
14 CLINICAL STUDIES
14.1 Efficacy in Neonates
Protective efficacy with ENGERIX-B has been demonstrated in a clinical trial in neonates at high risk of hepatitis B infection.6,7 Fifty-eight neonates born of mothers who were both HBsAg-positive and hepatitis B “e” antigen (HBeAg)-positive were given ENGERIX-B (10 mcg/0.5 mL) at 0, 1, and 2 months, without concomitant hepatitis B immune globulin (HBIG). Two infants became chronic carriers in the 12-month follow-up period after initial inoculation. Assuming an expected carrier rate of 70%, the protective efficacy rate against the chronic carrier state during the first 12 months of life was 95%.
14.2 Efficacy and Immunogenicity in Specific Populations
Homosexual Men
ENGERIX-B (20 mcg/1 mL) given at 0, 1, and 6 months was evaluated in homosexual men aged 16 to 59 years. Four of 244 subjects became infected with hepatitis B during the period prior to completion of the 3-dose immunization schedule. No additional subjects became infected during the 18-month follow-up period after completion of the immunization course.
Adults with Chronic Hepatitis C
In a clinical trial of 67 adults aged 25 to 67 years with chronic hepatitis C, ENGERIX-B (20 mcg/1 mL) was given at 0, 1, and 6 months. Of the subjects assessed at Month 7 (n = 31), 100% responded with seroprotective titers. The geometric mean antibody titer (GMT) was 1,260 mIU/mL (95% Confidence Interval [CI]: 709, 2,237).
Adults on Hemodialysis
Hemodialysis patients given hepatitis B vaccines respond with lower titers, which remain at protective levels for shorter durations than in normal subjects. In a clinical trial of 56 adults who had been on hemodialysis for a mean period of 56 months, ENGERIX-B (40 mcg/2 mL given as two 1-mL doses) was given at 0, 1, 2, and 6 months. Two months after the fourth dose, 67% (29/43) of patients had seroprotective antibody levels (≥10 mIU/mL) and the GMT among seroconverters was 93 mIU/mL.
Adults with Type 2 Diabetes Mellitus
In a descriptive study, 674 adult subjects with type 2 diabetes (diagnosed within the preceding 5 years) or without type 2 diabetes were enrolled and stratified by age and body mass index (BMI). The per-protocol immunogenicity cohort included 378 diabetic subjects and 189 matched control subjects who received ENGERIX-B (20 mcg/1 mL) at 0, 1, and 6 months. Among these subjects, the mean age was 54 years (range: 20 to 82 years); mean BMI was 32 kg/m2 (range: 17 to 64 kg/m2); 51% were male; 88% were white, 3% were American Indian or Alaskan Native, 3% were black, 2% were Asian, 4% were other racial groups; 2% were Hispanic or Latino.
The overall seroprotection rates (1 month after the third dose) were 75% (95% CI: 71, 80) in patients with diabetes and 82% (95% CI: 76, 87) in control subjects. The seroprotection rates in those with diabetes aged 20 to 39 years, 40 to 49 years, 50 to 59 years, and at least 60 years were 89%, 81%, 83%, and 58%, respectively. The seroprotection rates in those without diabetes in these same age-groups were 100%, 86%, 82%, and 70%, respectively. Subjects with diabetes and a BMI of at least 30 kg/m2 had a seroprotection rate of 72% compared with 80% in diabetic subjects with lower BMIs. In control subjects, seroprotection rates were 82% in those with a BMI of at least 30 kg/m2 and 83% in those with lower BMIs.
14.3 Immunogenicity in Neonates
In clinical studies, neonates were given ENGERIX-B (10 mcg/0.5 mL) at age 0, 1, and 6 months or at age 0, 1, and 2 months. The immune response to vaccination was evaluated in sera obtained 1 month after the third dose of ENGERIX-B.
Among infants administered ENGERIX-B at age 0, 1, and 6 months, 100% of evaluable subjects (n = 52) seroconverted by Month 7. The GMT was 713 mIU/mL. Of these, 97% had seroprotective levels (≥10 mIU/mL).
Among infants enrolled (n = 381) to receive ENGERIX-B at age 0, 1, and 2 months, 96% had seroprotective levels (≥10 mIU/mL) by Month 4. The GMT among seroconverters (n = 311) (antibody titer ≥1 mIU/mL) was 210 mIU/mL. A subset of these children received a fourth dose of ENGERIX-B at age 12 months. One month following this dose, seroconverters (n = 126) had a GMT of 2,941 mIU/mL.
14.4 Immunogenicity in Children and Adults
Persons Aged 6 Months through 10 Years
In clinical trials, children (N = 242) aged 6 months through 10 years were given ENGERIX-B (10 mcg/0.5 mL) at 0, 1, and 6 months. One to 2 months after the third dose, the seroprotection rate was 98% and the GMT of seroconverters was 4,023 mIU/mL.
Persons Aged 5 through 16 Years
In a separate clinical trial including both children and adolescents aged 5 through 16 years, ENGERIX-B (10 mcg/0.5 mL) was administered at 0, 1, and 6 months (n = 181) or 0, 12, and 24 months (n = 161). Immediately before the third dose of vaccine, seroprotection was achieved in 92.3% of subjects vaccinated on the 0-, 1-, and 6-month schedule and 88.8% of subjects on the 0-, 12-, and 24-month schedule (GMT: 118 mIU/mL versus 162 mIU/mL, respectively, P = 0.18). One month following the third dose, seroprotection was achieved in 99.5% of children vaccinated on the 0-, 1-, and 6-month schedule compared with 98.1% of those on the 0-, 12-, and 24-month schedule. GMTs were higher (P = 0.02) for children receiving vaccine on the 0-, 1-, and 6-month schedule compared with those on the 0-, 12-, and 24-month schedule (5,687 mIU/mL versus 3,159 mIU/mL, respectively).
Persons Aged 11 through 19 Years
In clinical trials with healthy adolescent subjects aged 11 through 19 years, ENGERIX-B (10 mcg/0.5 mL) given at 0, 1, and 6 months produced a seroprotection rate of 97% at Month 8 (n = 119) with a GMT of 1,989 mIU/mL (n = 118, 95% CI: 1,318, 3,020). Immunization with ENGERIX-B (20 mcg/1 mL) at 0, 1, and 6 months produced a seroprotection rate of 99% at Month 8 (n = 122) with a GMT of 7,672 mIU/mL (n = 122, 95% CI: 5,248, 10,965).
Persons Aged 16 through 65 Years
Clinical trials in healthy adult and adolescent subjects (aged 16 through 65 years) have shown that following a course of 3 doses of ENGERIX-B (20 mcg/1 mL) given at 0, 1, and 6 months, the seroprotection (antibody titers ≥10 mIU/mL) rate for all individuals was 79% at Month 6 (5 months after second dose) and 96% at Month 7 (1 month after third dose); the GMT for seroconverters was 2,204 mIU/mL at Month 7 (n = 110).
An alternate 3-dose schedule (20 mcg/1 mL given at 0, 1, and 2 months) designed for certain populations (e.g., individuals who have or might have been recently exposed to the virus and travelers to high-risk areas) was also evaluated. At Month 3 (1 month after third dose), 99% of all individuals were seroprotected and remained protected through Month 12. On the alternate schedule, a fourth dose of ENGERIX-B (20 mcg/1 mL) at 12 months produced a GMT of 9,163 mIU/mL at Month 13 (1 month after fourth dose) (n = 373).
Persons Aged 40 Years and Older
Among subjects aged 40 years and older given ENGERIX-B (20 mcg/1 mL) at 0, 1, and 6 months, the seroprotection rate 1 month after the third dose was 88% and the GMT for seroconverters was 610 mIU/mL (n = 50). In adults aged older than 40 years, ENGERIX-B produced anti-HBsAg antibody titers that were lower than those in younger adults.
14.5 Interchangeability with Other Hepatitis B Vaccines
A controlled study (N = 48) demonstrated that completion of a course of immunization with 1 dose of ENGERIX-B (20 mcg/1 mL) at Month 6 following 2 doses of RECOMBIVAX HB [Hepatitis B Vaccine (Recombinant)] (10 mcg) at Months 0 and 1 produced a similar GMT (4,077 mIU/mL) to immunization with 3 doses of RECOMBIVAX HB (10 mcg) at Months 0, 1, and 6 (GMT: 2,654 mIU/mL). Thus, ENGERIX-B can be used to complete a vaccination course initiated with RECOMBIVAX HB.8
-
15 REFERENCES
- 1.
- Centers for Disease Control and Prevention. Hepatitis B. In: Atkinson W, Wolfe C, Humiston S, Nelson R, eds. Epidemiology and Prevention of Vaccine-Preventable Diseases. 6th ed. Atlanta, GA: Public Health Foundation; 2000:207-229.
- 2.
- Centers for Disease Control and Prevention. A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Part 1: Immunization of Infants, Children, and Adolescents, MMWR. 2005;54(RR-16):1-23.
- 3.
- Ascherio A, Zhang SM, Hernán MA, et al. Hepatitis B vaccination and the risk of multiple sclerosis. N Engl J Med. 2001;344(5):327-332.
- 4.
- Confavreux C, Suissa S, Saddier P, et al. Vaccination and the risk of relapse in multiple sclerosis. N Engl J Med. 2001-344(5):319-326.
- 5.
- Centers for Disease Control and Prevention. A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Part 2: Immunization of Adults, MMWR. 2006;55(RR-16):1-25.
- 6.
- André FE, Safary A. Clinical experience with a yeast-derived hepatitis B vaccine. In: Zuckerman AJ, ed. Viral Hepatitis and Liver Disease. New York, NY: Alan R Liss, Inc.; 1988:1025-1030.
- 7.
- Poovorawan Y, Sanpavat S, Pongpunlert W, et al. Protective efficacy of a recombinant DNA hepatitis B vaccine in neonates of HBe antigen-positive mothers. JAMA. 1989;261(22):3278-3281.
- 8.
- Bush LM, Moonsammy GI, Boscia JA. Evaluation of initiating a hepatitis B vaccination schedule with one vaccine and completing it with another. Vaccine. 1991;9(11):807-809.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ENGERIX‑B (preservative-free formulation) is available in prefilled disposable TIP-LOK syringes (Luer Lock syringes) packaged without needles (pediatric/adolescent and adult doses) and single-dose vials (adult dose only). TIP-LOK syringes are to be used with Luer Lock compatible needles. The tip cap and rubber plunger stopper of the prefilled syringe are not made with natural rubber latex. The vial stoppers are not made with natural rubber latex.
10 mcg/0.5 mL Pediatric/Adolescent Dose
NDC 58160-820-43 Syringe in Package of 10: NDC 58160-820-52
20 mcg/mL Adult Dose
NDC 58160-821-01 Vial in Package of 10: NDC 58160-821-11
NDC 58160-821-43 Syringe in Package of 10: NDC 58160-821-52
Store refrigerated between 2° and 8°C (36° and 46°F). Do not freeze; discard if product has been frozen. Do not dilute to administer.
-
17 PATIENT COUNSELING INFORMATION
- •
- Inform vaccine recipients and parents or guardians of the potential benefits and risks of immunization with ENGERIX-B.
- •
- Emphasize, when educating vaccine recipients and parents or guardians regarding potential side effects, that ENGERIX-B contains non-infectious purified HBsAg and cannot cause hepatitis B infection.
- •
- Instruct vaccine recipients and parents or guardians to report any adverse events to their healthcare provider.
- •
- Give vaccine recipients and parents or guardians the Vaccine Information Statements, which are required by the National Childhood Vaccine Injury Act of 1986 to be given prior to immunization. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
ENGERIX-B, TWINRIX, and TIP-LOK are trademarks owned by or licensed to the GSK group of companies. The other brand listed is a trademark owned by or licensed to the respective owner and is not owned by or licensed to the GSK group of companies. The maker of this brand is not affiliated with and does not endorse the GSK group of companies or its products.
Manufactured by GlaxoSmithKline Biologicals
Rixensart, Belgium, U.S. License No. 1617
Distributed by GlaxoSmithKline
Durham, NC 27701
©2023 GSK group of companies or its licensor.
ENG:61PI
-
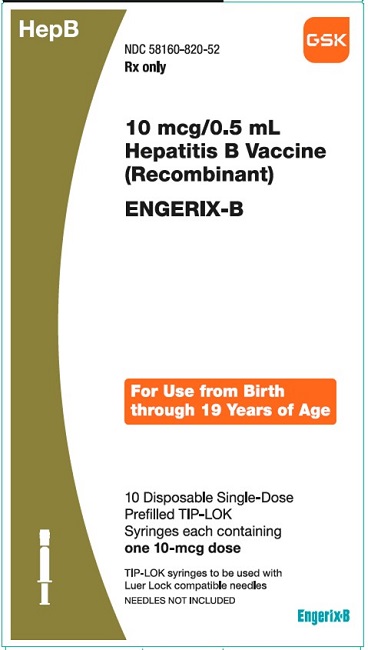
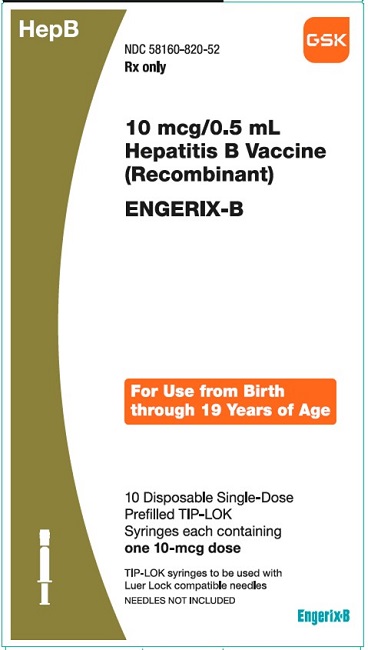
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 58160-820-52
ENGERIX-B
10 mcg/0.5mL
Hepatitis B Vaccine (Recombinant)
HepB
Rx only
For Use from Birth through 19 Years of Age
10 Disposable Single-Dose Prefilled TIP-LOK Syringes each containing one 10-mcg dose
TIP-LOK syringes to be used with Luer Lock compatible needles
NEEDLES NOT INCLUDED
GSK
Engerix-B
Made in Belgium
©2023 GSK group of companies or its licensor.
Rev.10/23
516604
-
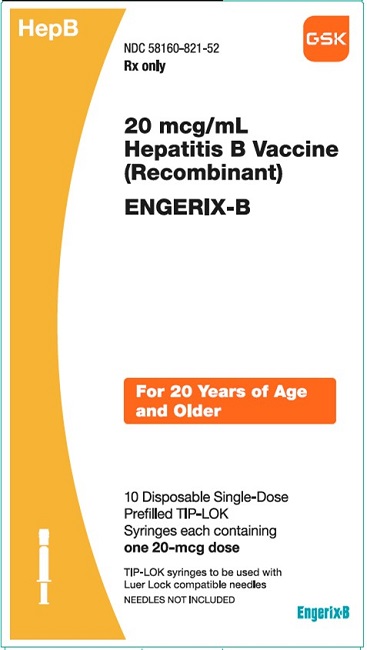
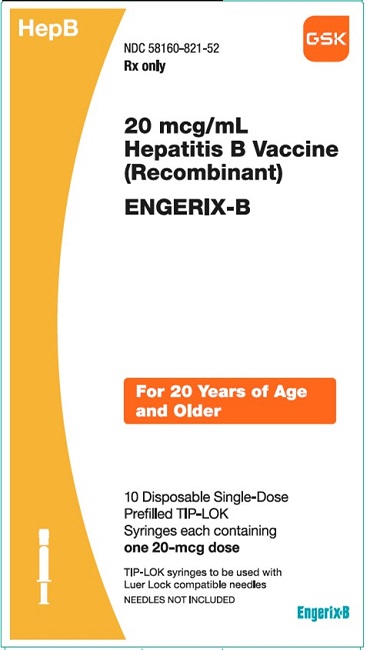
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 58160-821-52
ENGERIX-B
20 mcg/mL
Hepatitis B Vaccine (Recombinant)
HepB
Rx only
For 20 AYears of Age and Older
10 Disposable Single-Dose Prefilled TIP-LOK Syringes each containing one 20-mcg dose
TIP-LOK syringes to be used with Luer Lock compatible needles
NEEDLES NOT INCLUDED
GSK
Engerix-B
Made in Belgium
©2023 GSK group of companies or its licensor.
Rev.10/23
516603
-
INGREDIENTS AND APPEARANCE
ENGERIX-B
hepatitis b vaccine (recombinant) injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:58160-820 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS B VIRUS SUBTYPE ADW2 HBSAG SURFACE PROTEIN ANTIGEN (UNII: 9GCJ1L5D1P) (HEPATITIS B VIRUS SUBTYPE ADW2 HBSAG SURFACE PROTEIN ANTIGEN - UNII:9GCJ1L5D1P) HEPATITIS B VIRUS SUBTYPE ADW2 HBSAG SURFACE PROTEIN ANTIGEN 10 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58160-820-52 10 in 1 CARTON 1 NDC:58160-820-43 0.5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103239 04/25/2007 ENGERIX-B
hepatitis b vaccine (recombinant) injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:58160-821 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS B VIRUS SUBTYPE ADW2 HBSAG SURFACE PROTEIN ANTIGEN (UNII: 9GCJ1L5D1P) (HEPATITIS B VIRUS SUBTYPE ADW2 HBSAG SURFACE PROTEIN ANTIGEN - UNII:9GCJ1L5D1P) HEPATITIS B VIRUS SUBTYPE ADW2 HBSAG SURFACE PROTEIN ANTIGEN 20 ug in 1 mL Inactive Ingredients Ingredient Name Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58160-821-11 10 in 1 CARTON 1 NDC:58160-821-01 1 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC:58160-821-52 10 in 1 CARTON 2 NDC:58160-821-43 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:58160-821-34 1 in 1 CARTON 3 NDC:58160-821-05 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103239 03/28/2007 Labeler - GlaxoSmithKline Biologicals SA (372748392)