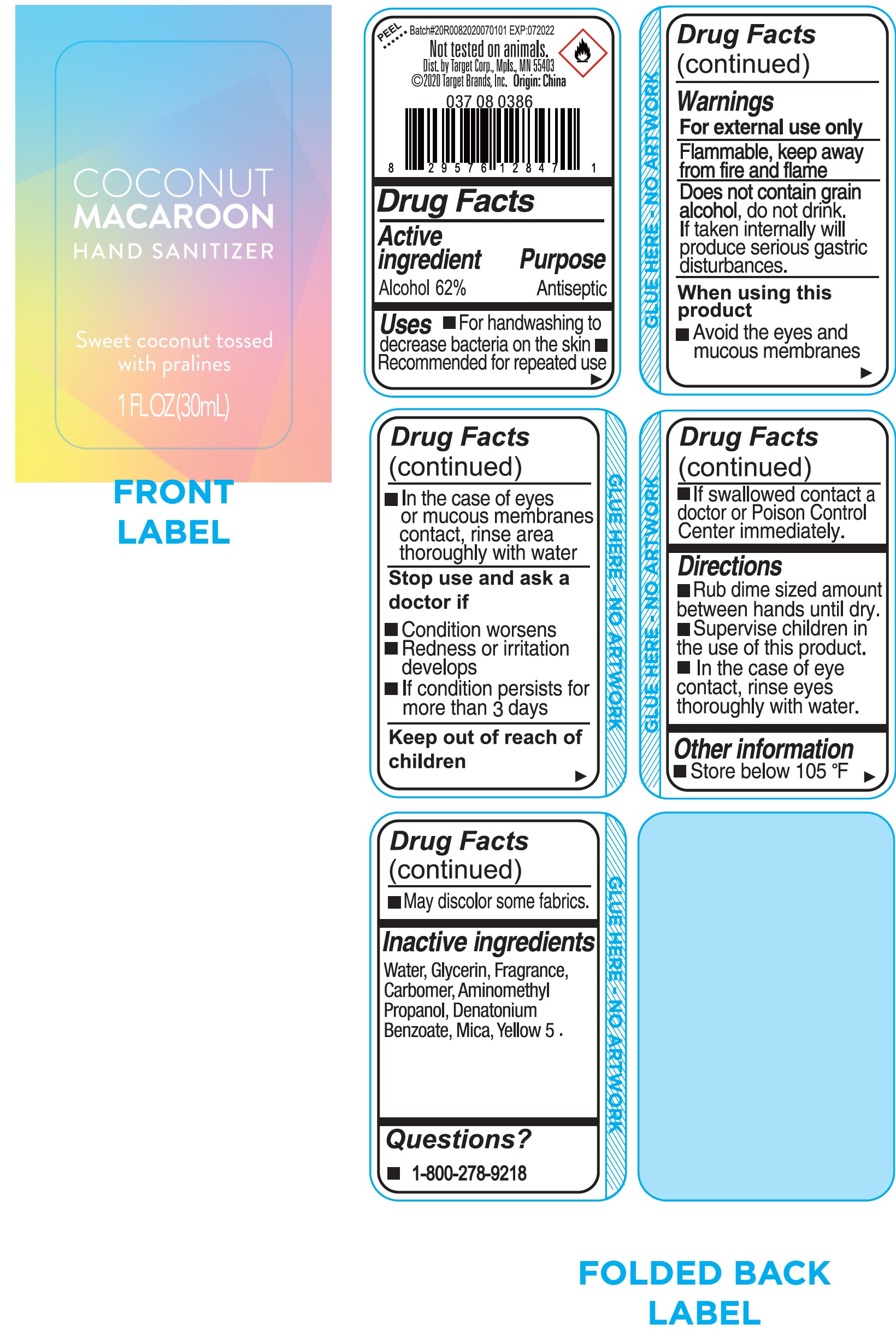
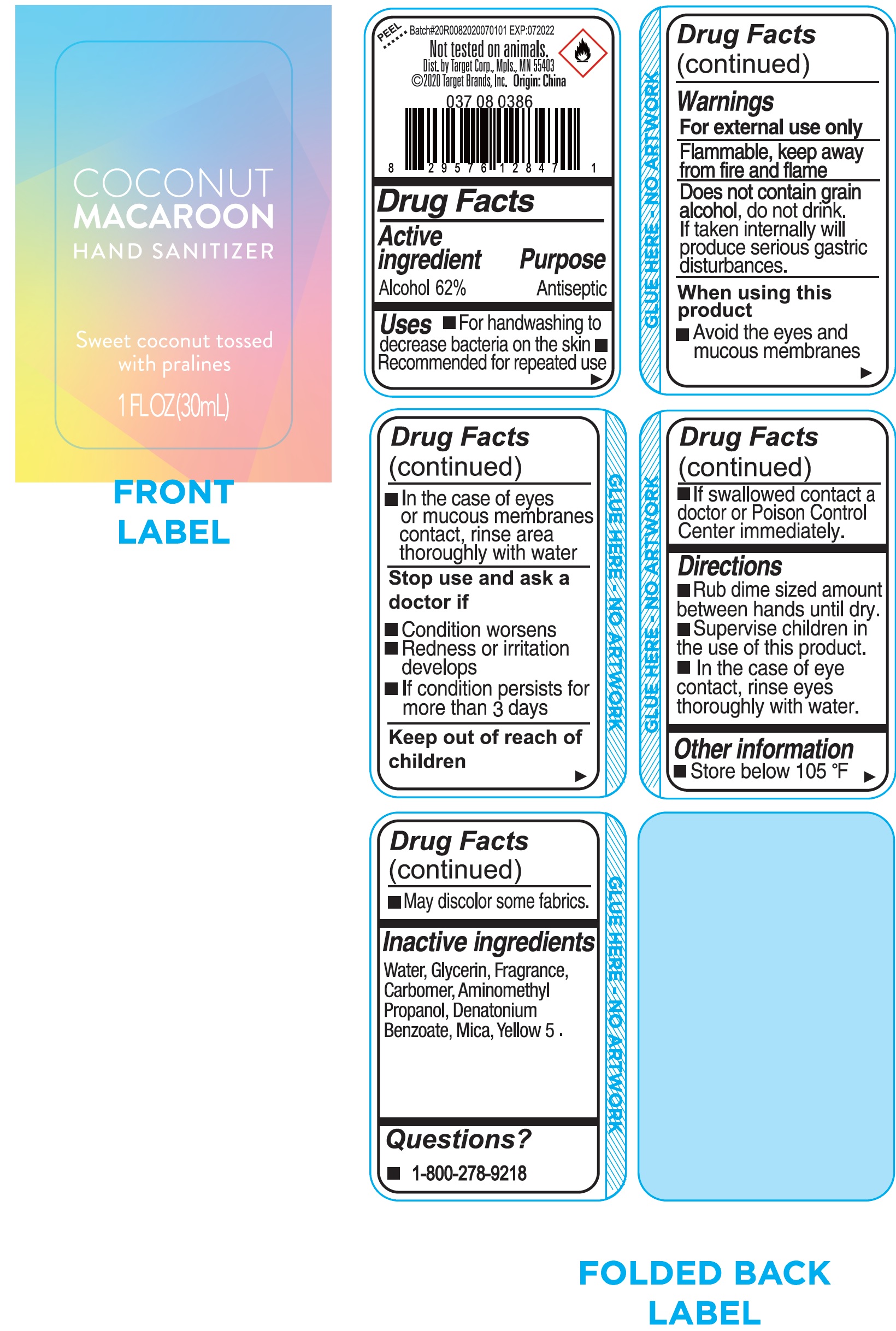
Label: COCONUT MACAROON HAND SANITIZER- alcohol gel
- NDC Code(s): 11673-780-00
- Packager: Target Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
-
Warnings
For external use only
Flammable, keep away from fire and flame
do not drink. If taken internally will produce serious gastric disturbance. Does not contain grain alcohol,
When using this product
- Avoid the eyes and mucous membranes
- In the case of eyes or mucous membranes contact, rinse area thoroughly with water
- Directions
- Other information
- Inactive ingredients
- Questions?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
COCONUT MACAROON HAND SANITIZER
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11673-780 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.62 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) MICA (UNII: V8A1AW0880) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-780-00 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/28/2020 Labeler - Target Corporation (006961700) Establishment Name Address ID/FEI Business Operations Bath Concept Cosmetics (Dongguan) Co., Ltd 529623933 manufacture(11673-780)