Label: SALONPAS ARTHRITIS PAIN- menthol, methyl salicylate patch
-
NDC Code(s):
46581-680-01,
46581-680-03,
46581-680-05,
46581-680-15, view more46581-680-20, 46581-680-99
- Packager: Hisamitsu Pharmaceutical Co., Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 2, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each patch)
- Purpose
- Uses
-
Warnings
For external use only
Stomach bleeding warning:
This product contains an NSAID, which may cause stomach bleeding. The chance is small but higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing an NSAID [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- on the face or rashes
- on wouds or damaged skin
- if allergic to aspirin or other NSAIDs
- with a heating pad
- when sweating (such as from exercise or heat)
- any patch from a pouch that has been open for 14 or more days
- right before or after heart surgery
Ask a doctor before use if
- you are allergic to topical products
- the stomach bleeding warning applies to you
- you are taking a diuretic
- you have high blood pressure, heart disease, or kidney disease
When using this product
- wash hands after applying or removing patch. Avoid contact with eyes. If eye contact occurs, rinse thoroughly with water.
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
Stop use and ask a doctor if
- stomach pain or upset gets worse or lasts
- you feel faint, vomit blood, or have bloody or black stools. These are signs of stomach beeding.
- rash, itching or skin irritaion develops
- condition worsens
- symptoms last for more than 3 days
- symptoms clear up and occur again within a few days
-
Directions
Adults 18 years and older:
- only use one patch at a time
- clean and dry affected area
- remove patch from backing film and apply to skin (see illustration)
- apply one patch to the affected area and leave in place for up to 8 to 12 hours
- if pain lasts after using the first patch, a second patch may be applied for up to another 8 to 12 hours
- do not use more than 2 patches per day
- do not use for more than 3 days in a row
- the used patch should be removed from the skin when a new one is applied
Children under 18 years of age: do not use; this product has not been shown to work in children
- Other information
- Inactive inredients
- Questions or comments?
-
Principal Display Panel
Hisamitsu
NDC#46581-680-20
for temporary relief of mild to moderate pain
Arthritis
Joint Pain
Sprains
StrainsSalonpas
Arthritis Pain Patch
APPLY FOR 8-12 HOURS
Menthol 3%
Methyl Salicylate 10%
Topical Analgesic
FDA APPROVED NON-PRESCRIPTION PAIN RELIEVING PATCH
EFFECTIVENESS CONFIRMED IN CLINICAL TRIAL
20 pathces
2 3/4" X 3 15/16"(7cm X 10cm)
Package not child resistant.
Keep our of reach of children.
* DO NOT USE MORE THAN ONE SALONPAS PATCH AT A TIME. SEE DIRECTIONS

-
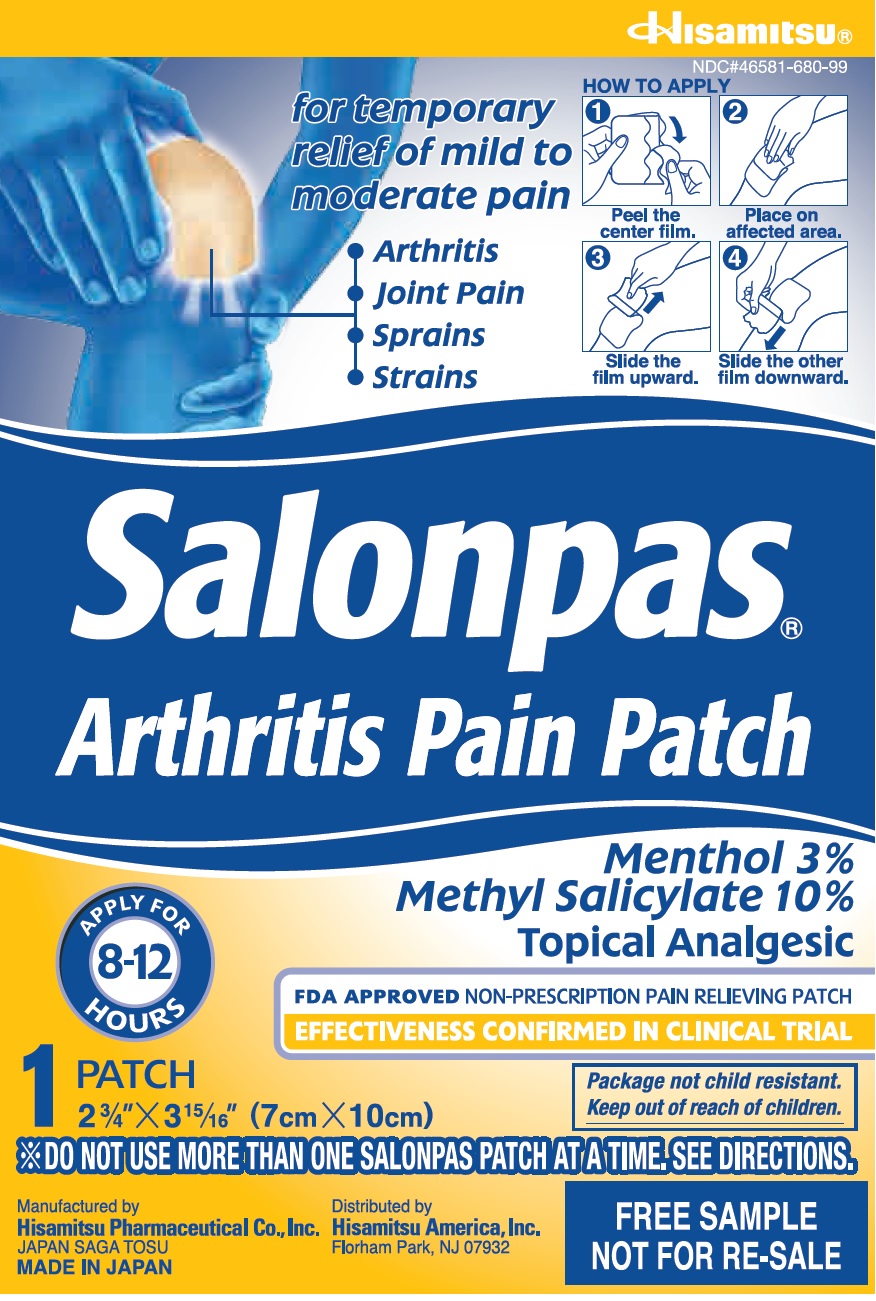
Principal Display Panel
NDC#46581-680-99
for temporary relief of mild to moderate pain
Arthritis
Joint Pain
Sprains
StrainsHOW TO APPLY
1 Peel the center film.
2 Place on affected area.
3 Slide the film upward.
4 Slide the other film downward.Salonpas
Arthritis Pain PatchAPPLY FOR 8-12 HOURS
Menthol 3%
Methyl Salicylate 10%
Topical AnalgesicFDA APPROVED NON-PRESCRIPTION PAIN RELIEVING PATCH
EFFECTIVENESS CONFIRMED IN CLINICAL TRIAL1 patch
2 3/4" X 3 15/16" (7cm X 10cm)Package not child resistant.
Keep out of reach of children.*DO NOT USE MORE THAN ONE SALONPAS PATCH AT A TIME, SEE DIRECTIONS
Manufactured by
Hisamitsu Pharmaceutical Co., Inc.
JAPAN SAGA TOSU
MADE IN JAPANDistributed by
Hisamitsu America, Inc.
Florham Park, NJ 07932FREE SAMPLE
NOT FOR RE-SALE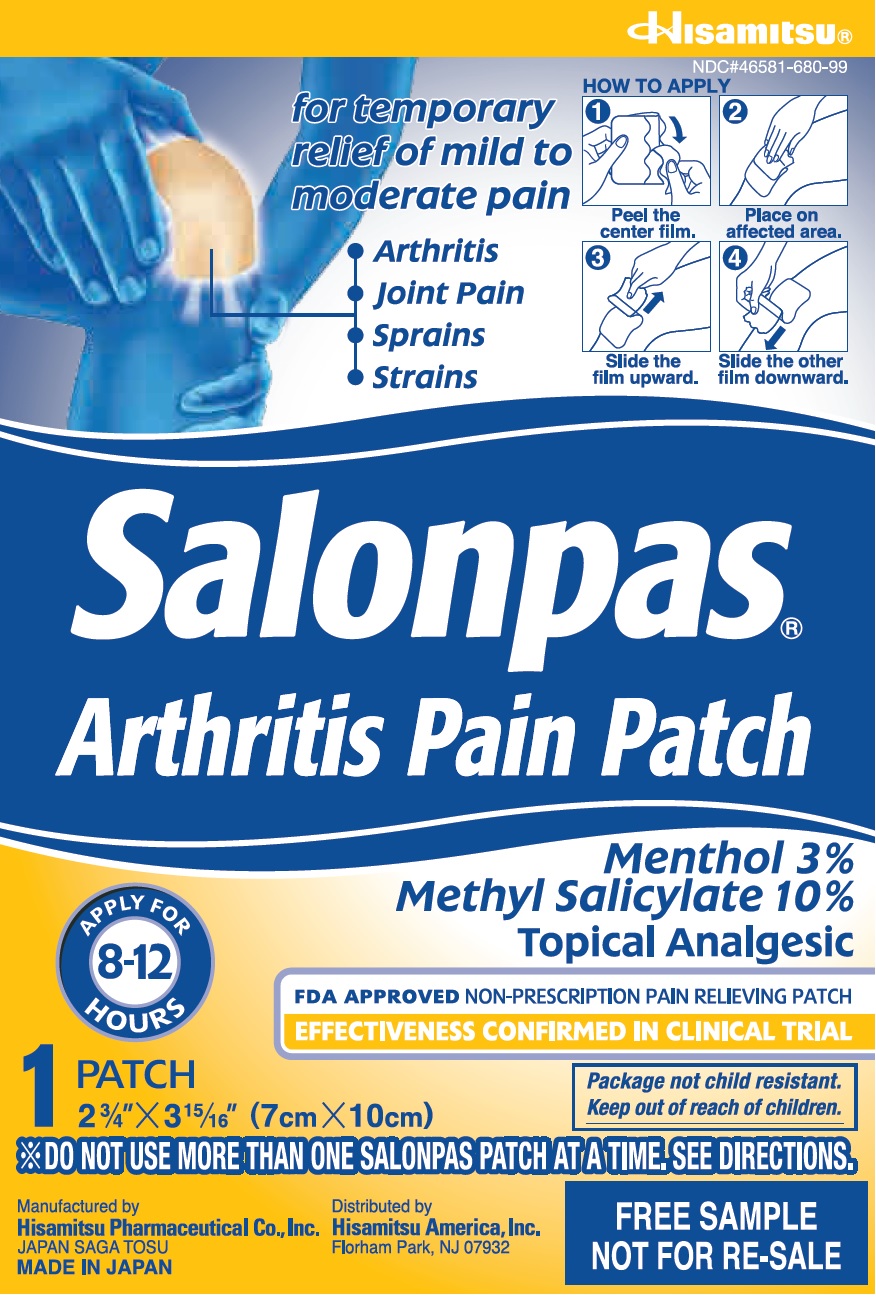
-
INGREDIENTS AND APPEARANCE
SALONPAS ARTHRITIS PAIN
menthol, methyl salicylate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46581-680 Route of Administration TOPICAL, PERCUTANEOUS, TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 31.5 mg METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 105 mg Inactive Ingredients Ingredient Name Strength ALUMINUM SILICATE (UNII: T1FAD4SS2M) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46581-680-15 3 in 1 BOX 06/01/2008 1 5 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:46581-680-05 1 in 1 BOX 05/01/2008 2 5 in 1 POUCH; Type 0: Not a Combination Product 3 NDC:46581-680-20 4 in 1 BOX 11/01/2022 3 5 in 1 POUCH; Type 0: Not a Combination Product 4 NDC:46581-680-03 3 in 1 POUCH; Type 0: Not a Combination Product 09/01/2008 5 NDC:46581-680-01 1 in 1 POUCH; Type 0: Not a Combination Product 05/01/2008 6 NDC:46581-680-99 1 in 1 POUCH; Type 0: Not a Combination Product 05/01/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022029 04/25/2008 Labeler - Hisamitsu Pharmaceutical Co., Inc. (690539713)