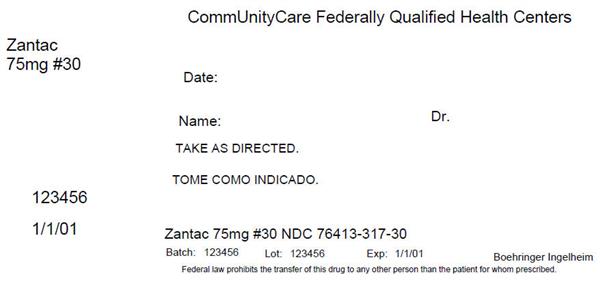
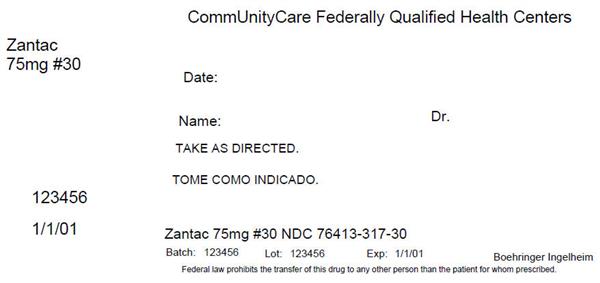
Label: ZANTAC- ranitidine tablet, coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 76413-317-30 - Packager: Central Texas Community Health Centers
- This is a repackaged label.
- Source NDC Code(s): 0597-0122
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 26, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Uses
- Warnings
- Do not use
-
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- Stop use and ask a doctor if
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water
- to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn
- can be used up to twice daily (do not take more than 2 tablets in 24 hours)
- do not chew tablet
- children under 12 years: ask a doctor
- adults and children 12 years and over:
- Other information
- Inactive ingredients
- Questions?
-
SPL UNCLASSIFIED SECTION
Read the directions, consumer information leaflet and warnings before use. Keep the carton. It contains important information.
Distributed by: Boehringer Ingelheim (BI) Consumer Health Care Products
Division of BI Pharmaceuticals, Inc., Ridgefield, CT 06877
© 2016, BI Pharmaceuticals, Inc. All rights reserved.
Product of Spain. Manufactured in Mexico. - INDICATIONS & USAGE
- HOW SUPPLIED
- Zantac
-
INGREDIENTS AND APPEARANCE
ZANTAC
ranitidine tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76413-317(NDC:0597-0122) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RANITIDINE HYDROCHLORIDE (UNII: BK76465IHM) (RANITIDINE - UNII:884KT10YB7) RANITIDINE 75 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) magnesium stearate (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) ferric oxide red (UNII: 1K09F3G675) titanium dioxide (UNII: 15FIX9V2JP) triacetin (UNII: XHX3C3X673) Product Characteristics Color PINK Score no score Shape PENTAGON (5 sided) Size 3mm Flavor Imprint Code Z;75 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76413-317-30 3 in 1 CARTON 12/21/2006 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020520 12/21/2006 Labeler - Central Texas Community Health Centers (079674019) Establishment Name Address ID/FEI Business Operations Travis County Healthcare District 797039398 RELABEL(76413-317) , REPACK(76413-317)