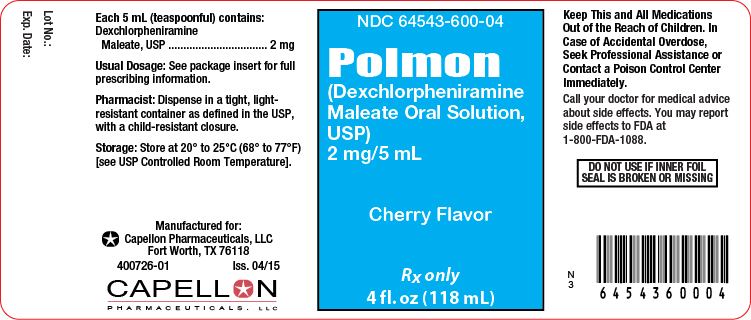
Label: POLMON- dexchlorpheniramine maleate solution
- NDC Code(s): 64543-600-04, 64543-600-16
- Packager: Capellon Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Each 5 mL (teaspoonful) contains:
Dexchlorpheniramine Maleate, USP ............. 2 mg
Dexchlorpheniramine Maleate, USP, an antihistamine agent, is a white, odorless crystalline powder that is freely soluble in water. The molecular formula is C 16H 19ClN 2 • C 4H 4O 4, designated chemically as (+)-2-[p-Chloro-α-[2-(dimethylamino)ethyl]benzyl]pyridine maleate (1:1).
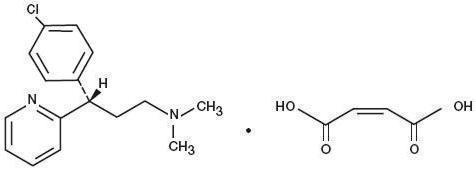
M.W. = 390.86
- INACTIVE INGREDIENTS
- CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
Perennial and seasonal allergic rhinitis
Vasomotor rhinitis
Allergic conjunctivitis due to inhalant allergens and foods
Mild, uncomplicated allergic skin manifestations of urticaria and angioedema
Amelioration of allergic reactions to blood or plasma
DermographismAs therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
-
CONTRAINDICATIONS
Use in Newborn or Premature Infants
This drug should not be used in newborn or premature infants.Use in Nursing Mothers
Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
Use in Lower Respiratory Disease
Antihistamines should NOT be used to treat lower respiratory tract symptoms including asthma.
Antihistamines are also contraindicated in the following conditions:
-
Hypersensitivity to dexchlorpheniramine maleate or other antihistamines of similar chemical structure
Monoamine oxidase inhibitor therapy (See Drug Interaction section)
-
-
WARNINGS
Antihistamines should be used with considerable caution in patients with:
-
Narrow angle glaucoma
Stenosing peptic ulcer
Pyloroduodenal obstruction
Symptomatic prostatic hypertrophy
Bladder neck obstruction
Use in Children:
In infants and children, especially, antihistamines in overdosage may cause hallucinations, convulsions, or death.
As in adults, antihistamines may diminish mental alertness in children. In the young child, particularly, they may produce excitation.
Use in Pregnancy:
Experience with this drug in pregnant women is inadequate to determine whether there exists a potential for harm to the developing fetus.
Use with CNS Depressants:
POLMON Oral Solution has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc.).
Use in Activities Requiring Mental Alertness:
Patients should be warned about engaging in activities requiring mental alertness such as driving a car or operating appliances, machinery, etc.
Use in the Elderly (approximately 60 years or older):
Antihistamines are more likely to cause dizziness, sedation, and hypotension in elderly patients.
-
- PRECAUTIONS
-
ADVERSE REACTIONS
- General: Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose and the throat.
- Cardiovascular System: Hemolytic anemia, thrombocytopenia, agranulocytosis.
- Hematologic System: Hemolytic anemia, thrombocytopenia, agranulocytosis.
- Nervous System: Sedation, sleepiness, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesias, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, hysteria, neuritis, convulsions.
- G.I. System: Epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation.
- G.U. System: Urinary frequency, difficult urination, urinary retention, early menses.
- Respiratory System: Thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness.
-
OVERDOSAGE
Antihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in children. Atropine-like signs and symptoms—dry mouth, fixed, dilated pupils, flushing, and gastrointestinal symptoms may also occur.
If vomiting has not occurred spontaneously the patient should be induced to vomit. This is best done by having the patient drink a glass of water or milk after which the patient should be made to gag. Precautions against aspiration must be taken, especially in infants and children.
Saline cathartics, such as milk of magnesia, draw water into the bowel by osmosis and therefore, are valuable for their action in rapid dilution of bowel content.
Stimulants should not be used.
Vasopressors may be used to treat hypotension.
-
DOSAGE AND ADMINISTRATION
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT.
Recommended Dosage
Adults and Children 12 years of age and older: 2 mg (1 teaspoonful)
Children 6 to 11 years: 1 mg (1/2 teaspoonful)
Children 2 to 5 years: 0.5 mg (1/4 teaspoonful)
Doses are generally given every 4 to 6 hours.
- HOW SUPPLIED
-
Principal Display Panel-4 fl oz. Bottle
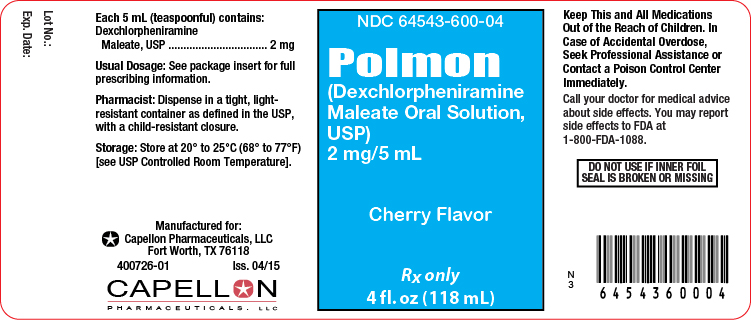
4 fl oz. Bottle Label
NDC 64543-600-04
POLMON
(dexchlorpheniramine maleate oral solution, USP)
2 mg per 5 mLRx Only
4 fl oz. (118 mL)
USUAL DOSAGE: See Package Insert for Complete Dosage Recommendations.
Dispense in a tight, light-resistant container with a child-resistant closure.WARNING: KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Tamper evident by foil seal under cap. Do not use if inner foil seal is broken or missing.
Manufactured for:
Capellon Pharmaceuticals, LLC
Fort Worth, TX 76118
16 fl oz. Bottle Label
NDC 64543-600-16
POLMON
(dexchlorpheniramine maleate oral solution, USP)
2 mg per 5 mLRx Only
16 fl oz. (473 mL)
USUAL DOSAGE: See Package Insert for Complete Dosage Recommendations.
Dispense in a tight, light-resistant container with a child-resistant closure.WARNING: KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Tamper evident by foil seal under cap. Do not use if inner foil seal is broken or missing.
Manufactured for:
Capellon Pharmaceuticals, LLC
Fort Worth, TX 76118 -
INGREDIENTS AND APPEARANCE
POLMON
dexchlorpheniramine maleate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64543-600 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXCHLORPHENIRAMINE MALEATE (UNII: B10YD955QW) (DEXCHLORPHENIRAMINE - UNII:3Q9Q0B929N) DEXCHLORPHENIRAMINE MALEATE 2 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL (UNII: L7T10EIP3A) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SUCROSE (UNII: C151H8M554) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64543-600-04 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/16/2018 10/05/2024 2 NDC:64543-600-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/16/2018 10/05/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202520 07/16/2018 10/05/2024 Labeler - Capellon Pharmaceuticals, LLC (124568093)