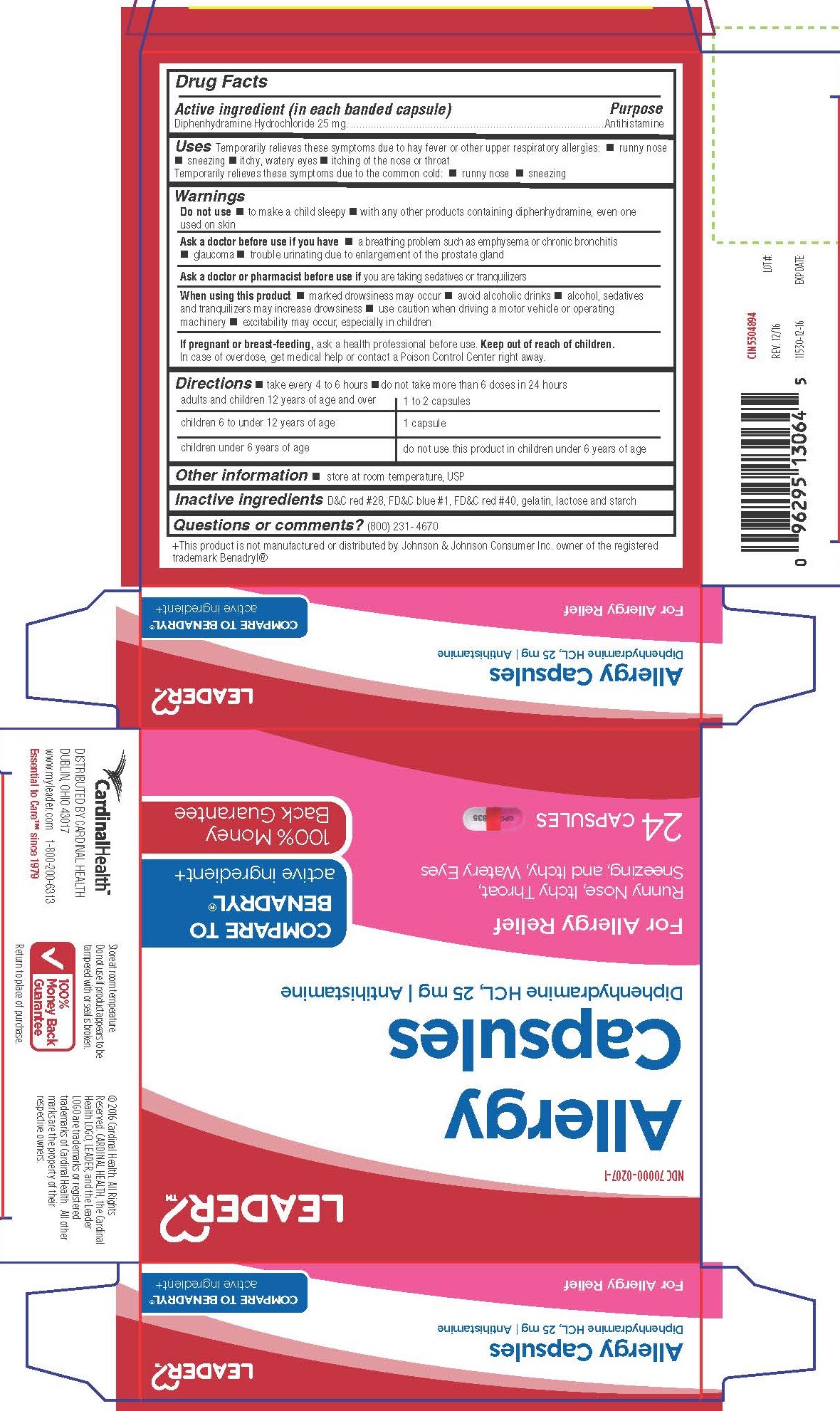
Label: DIPHENHYDRAMINE HYDROCHLORIDE capsule
- NDC Code(s): 70000-0207-1, 70000-0207-2
- Packager: Cardinal Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
-
Warnings
Do not use
- to make child sleepy
- with any other products containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to enlargement of the prostate gland
- Directions
- Other Information
- INACTIVE INGREDIENT
- Questions or Comments?
-
SPL UNCLASSIFIED SECTION
Distributed By Cardinal Health
Dublin, Ohio 43017
www.myleader.com
Essential to Care™ since 1979
+This product is not manufactured or distributed by Johnson & Johnson Consumer Inc. owner of the registered trademark Benadryl®
© 2016 Cardinal Health. All rights Reserved. Cardinal Health, the Cardinal Health Logo, Leadear and the Leader Logo are trademarks or registered trademarks of Cardinal Health. All other marks are property of their respective owners.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0207 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color pink (Half pink and half clear with white powder inside) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code CPC;835 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0207-1 2 in 1 CARTON 01/31/2017 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:70000-0207-2 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/13/2017 Labeler - Cardinal Health (063997360)