Label: NEST FRAGRANCES BAMBOO AND JASMINE- octinoxate and oxybenzone stick
NEST FRAGRANCES GINGER AND NEROLI- octinoxate and oxybenzone stick
NEST FRAGRANCES GRAPEFRUIT AND VERBENA- octinoxate and oxybenzone stick
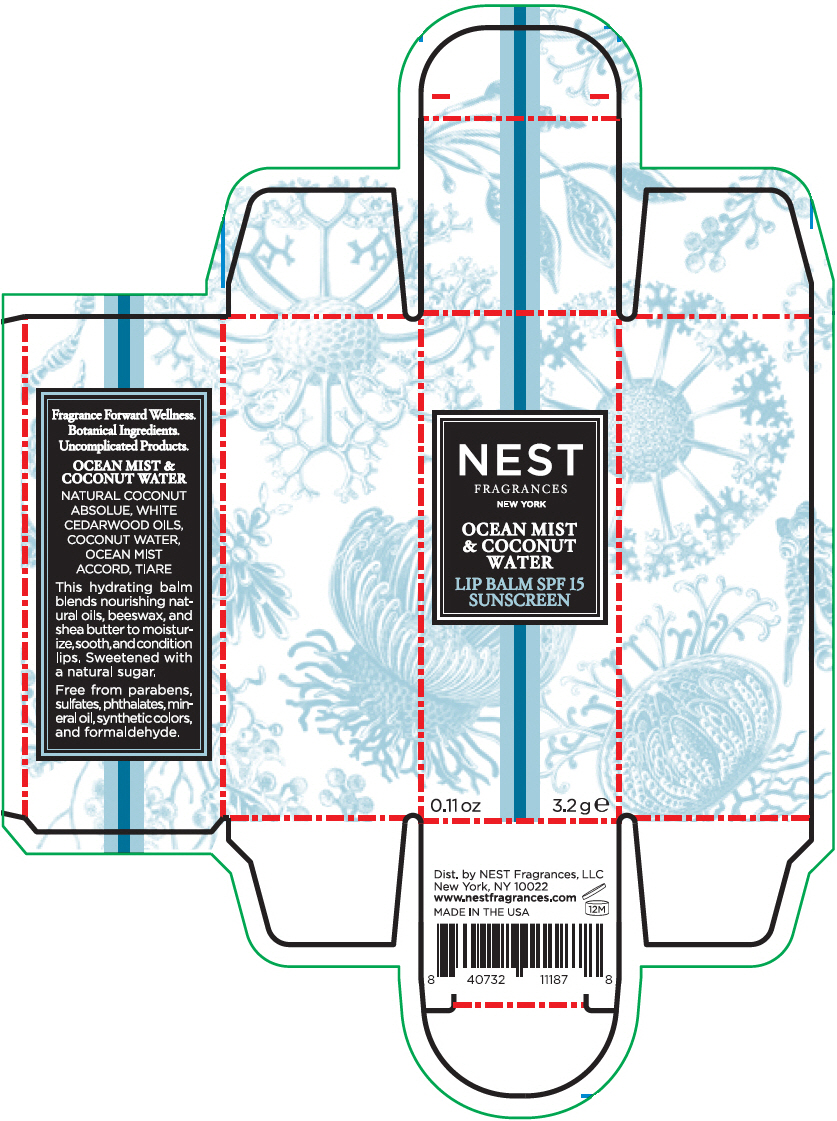
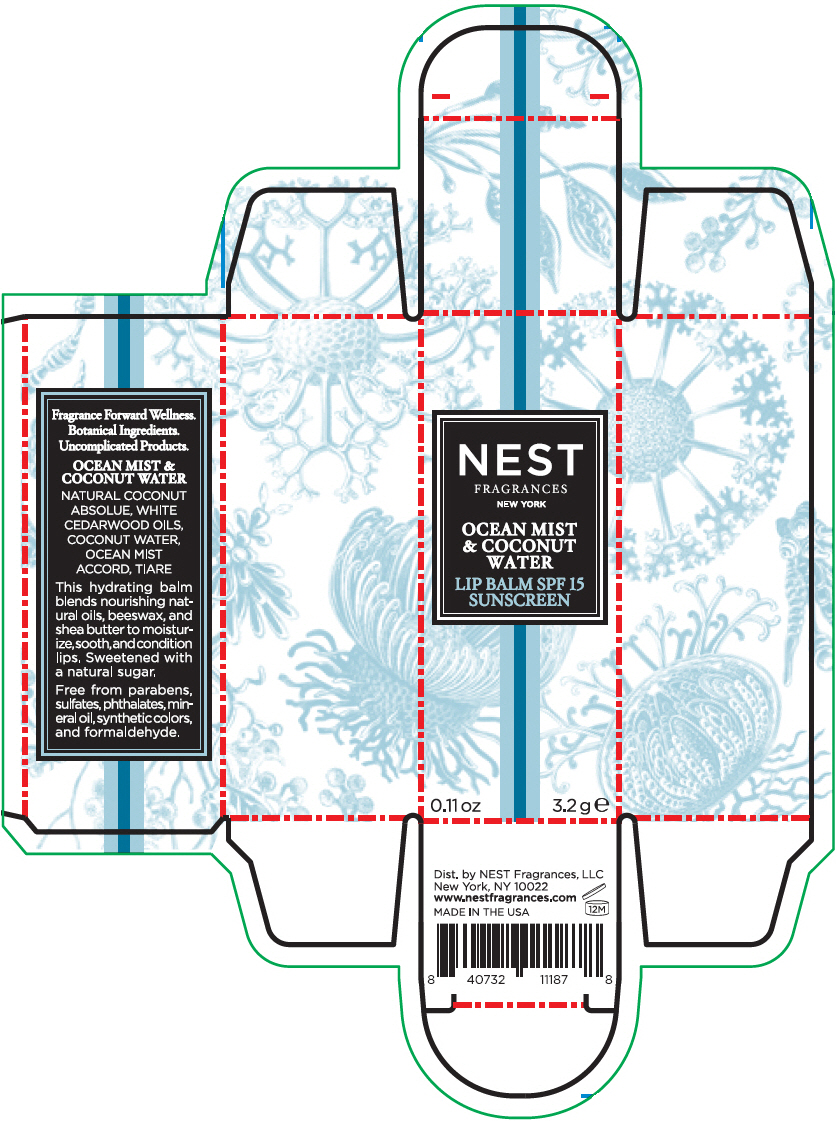
NEST FRAGRANCES OCEAN MIST AND COCONUT WATER- octinoxate and oxybenzone stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 72556-001-01, 72556-002-01, 72556-003-01, 72556-004-01 - Packager: Nest Fragrances LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 1, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
- Warnings
- Directions
- Other information
-
Inactive Ingredients
Beeswax (Cera Alba), Macadamia Ternifolia Seed Oil, Cocos Nucifera (Coconut) Oil, Vitis Vinifera (Grape) Seed Oil, Simmond- sia Chinensis (Jojoba) Seed Oil, Copernicia Cerifera (Carnauba) Wax, Prunus Armeniaca (Apricot) Kernel Oil, Shea Butter Ethyl Esters, Fragrance (Parfum), Erythritol, Flavor (Aroma), Tocopheryl Acetate, Rosma- rinus Officinalis (Rose- mary) Leaf Extract, Helianthus Annuus (Sunflower) Seed Oil.
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 3.2 g Tube Carton - BAMBOO & JASMINE
- PRINCIPAL DISPLAY PANEL - 3.2 g Tube Carton - GINGER & NEROLI
- PRINCIPAL DISPLAY PANEL - 3.2 g Tube Carton - GRAPEFRUIT & VERBENA
- PRINCIPAL DISPLAY PANEL - 3.2 g Tube Carton - OCEAN MIST & COCONUT WATER
-
INGREDIENTS AND APPEARANCE
NEST FRAGRANCES BAMBOO AND JASMINE
octinoxate and oxybenzone stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72556-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 75 mg in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 20 mg in 1 g Inactive Ingredients Ingredient Name Strength WHITE WAX (UNII: 7G1J5DA97F) MACADAMIA OIL (UNII: 515610SU8C) COCONUT OIL (UNII: Q9L0O73W7L) GRAPE SEED OIL (UNII: 930MLC8XGG) JOJOBA OIL (UNII: 724GKU717M) CARNAUBA WAX (UNII: R12CBM0EIZ) APRICOT KERNEL OIL (UNII: 54JB35T06A) Erythritol (UNII: RA96B954X6) SUNFLOWER OIL (UNII: 3W1JG795YI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72556-001-01 1 in 1 CARTON 12/15/2018 1 3.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 12/15/2018 NEST FRAGRANCES GINGER AND NEROLI
octinoxate and oxybenzone stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72556-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 75 mg in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 20 mg in 1 g Inactive Ingredients Ingredient Name Strength WHITE WAX (UNII: 7G1J5DA97F) MACADAMIA OIL (UNII: 515610SU8C) COCONUT OIL (UNII: Q9L0O73W7L) GRAPE SEED OIL (UNII: 930MLC8XGG) JOJOBA OIL (UNII: 724GKU717M) CARNAUBA WAX (UNII: R12CBM0EIZ) APRICOT KERNEL OIL (UNII: 54JB35T06A) Erythritol (UNII: RA96B954X6) SUNFLOWER OIL (UNII: 3W1JG795YI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72556-002-01 1 in 1 CARTON 12/15/2018 1 3.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 12/15/2018 NEST FRAGRANCES GRAPEFRUIT AND VERBENA
octinoxate and oxybenzone stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72556-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 75 mg in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 20 mg in 1 g Inactive Ingredients Ingredient Name Strength WHITE WAX (UNII: 7G1J5DA97F) MACADAMIA OIL (UNII: 515610SU8C) COCONUT OIL (UNII: Q9L0O73W7L) GRAPE SEED OIL (UNII: 930MLC8XGG) JOJOBA OIL (UNII: 724GKU717M) CARNAUBA WAX (UNII: R12CBM0EIZ) APRICOT KERNEL OIL (UNII: 54JB35T06A) Erythritol (UNII: RA96B954X6) SUNFLOWER OIL (UNII: 3W1JG795YI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72556-003-01 1 in 1 CARTON 12/15/2018 1 3.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 12/15/2018 NEST FRAGRANCES OCEAN MIST AND COCONUT WATER
octinoxate and oxybenzone stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72556-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 75 mg in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 20 mg in 1 g Inactive Ingredients Ingredient Name Strength WHITE WAX (UNII: 7G1J5DA97F) MACADAMIA OIL (UNII: 515610SU8C) COCONUT OIL (UNII: Q9L0O73W7L) GRAPE SEED OIL (UNII: 930MLC8XGG) JOJOBA OIL (UNII: 724GKU717M) CARNAUBA WAX (UNII: R12CBM0EIZ) APRICOT KERNEL OIL (UNII: 54JB35T06A) Erythritol (UNII: RA96B954X6) SUNFLOWER OIL (UNII: 3W1JG795YI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72556-004-01 1 in 1 CARTON 12/15/2018 1 3.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 12/15/2018 Labeler - Nest Fragrances LLC (363912010)