Label: SYSTANE COMPLETE- propylene glycol emulsion
-
NDC Code(s):
0065-0481-01,
0065-0481-10,
0065-0481-11,
0065-0481-55, view more0065-0481-72
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
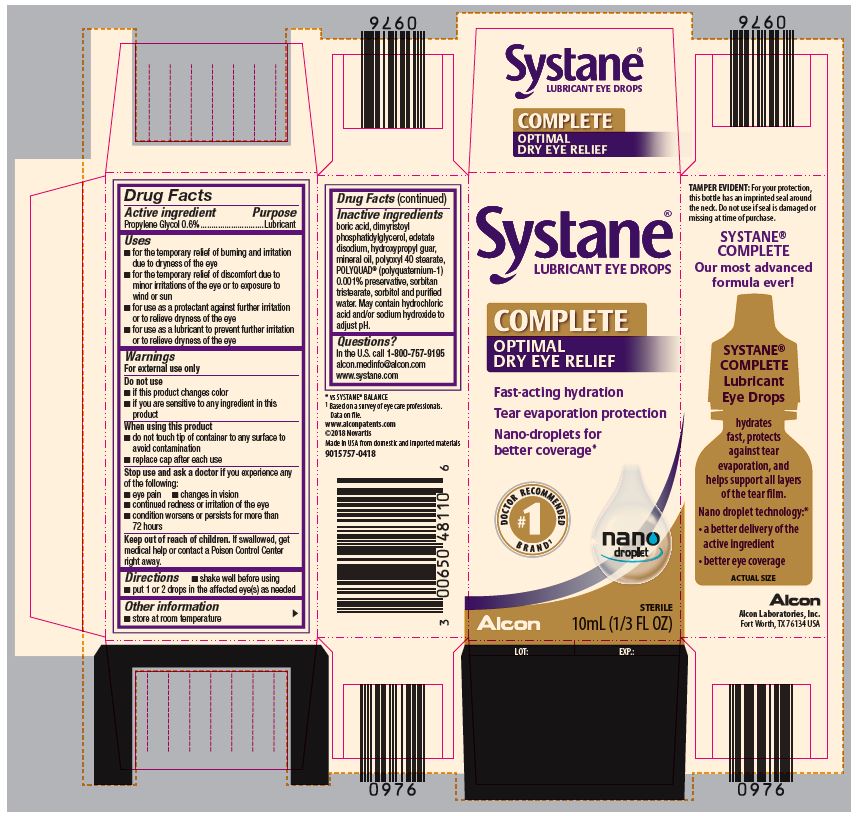
- ACTIVE INGREDIENT
-
Uses
- for the temporary relief of burning and irritation due to dryness of the eye
- for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
- for use as a protectant against further irritation or to relieve dryness of the eye
- for use as a lubricant to prevent further irritation or to relieve dryness of the eye
-
Warnings
For external use only
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
- Directions
- Other Information
- Inactive Ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL
Systane®
LUBRICANT EYE DROPS
COMPLETE
OPTIMAL DRY EYE RELIEF
Fast-acting hydration
Tear evaporation protection
Nano-droplets for better coverage*
#1 DOCTOR RECOMMENDED BRAND1
nano droplet
Alcon
STERILE
10mL (1/3 FL OZ)
LOT: EXP.:SIDE PANEL
TAMPER EVIDENT: For your protection,
this bottle has an imprinted seal around
the neck. Do not use if seal is damaged or
missing at time of purchase.
SYSTANE®
COMPLETE
Our most advanced
formula ever!
SYSTANE®
COMPLETE
Lubricant
Eye Drops
hydrates
fast, protects
against tear
evaporation, and
helps support all layers
of the tear film.
Nano droplet technology:*
● a better delivery of the
active ingredient
● better eye coverage
ACTUAL SIZE
Alcon
Alcon Laboratories, Inc.
Fort Worth, TX 76143 USA
* vs SYSTANE® BALANCE
1Based on a survey of eye care professionals.
Data on file.
www.alconpatents.com
Made in USA from domestic and imported materials
300048645-0621

Systane®
LUBRICANT EYE DROPS
COMPLETE
OPTIMAL DRY EYE RELIEF
Fast-acting hydration
Tear evaporation protection
Nano-droplets for better coverage*
#1 DOCTOR RECOMMENDED BRAND1
nano droplet
Alcon
STERILE
10mL (1/3 FL OZ)SIDE PANEL
TAMPER EVIDENT: For your protection, this bottle has an imprinted seal around the neck. Do not use if seal is damaged or missing at time of purchase.
Systane®COMPLETE
Our most advanced formula ever!
Systane® COMPLETE Lubricant Eye Drops hydrates fast, protects against tear evaporation, and helps support all layers of the tear film.
Nano droplet technology*
● a better delivery of the active ingredient
● better eye coverage
* vs Systane®BALANCE
1 Based on a survey of eye care professionals. Data on file.
www.alconpatents.com
©2018 Novartis
Made in USA from domestic and imported materials
9015757-0418
Alcon
Alcon Laboratories, Inc.
Fort Worth, TX 76143 USA
-
INGREDIENTS AND APPEARANCE
SYSTANE COMPLETE
propylene glycol emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0065-0481 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Propylene Glycol (UNII: 6DC9Q167V3) (Propylene Glycol - UNII:6DC9Q167V3) Propylene Glycol .06 mg in 1 mL Inactive Ingredients Ingredient Name Strength Boric Acid (UNII: R57ZHV85D4) Dimyristoylphosphatidylglycerol, Dl- (UNII: BI71WT9P3R) Edetate Disodium (UNII: 7FLD91C86K) Guar Gum (UNII: E89I1637KE) Mineral Oil (UNII: T5L8T28FGP) Polyoxyl 40 Stearate (UNII: 13A4J4NH9I) Sorbitan Tristearate (UNII: 6LUM696811) Sorbitol (UNII: 506T60A25R) Water (UNII: 059QF0KO0R) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Polidronium Chloride (UNII: 6716Z5YR3G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-0481-10 1 in 1 CARTON 01/18/2018 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:0065-0481-11 2 in 1 CARTON 01/18/2018 2 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 3 NDC:0065-0481-01 1 in 1 CARTON 01/18/2018 3 1.5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 4 NDC:0065-0481-55 1 in 1 CARTON 01/18/2018 4 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 5 NDC:0065-0481-72 3 in 1 CARTON 01/18/2018 5 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 01/18/2018 Labeler - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-0481)