Label: BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 CASHEW 3.5- titanium dioxide gel
BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 CINNAMON 10.5- titanium dioxide gel
BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 CEDAR 11- titanium dioxide gel
BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 MAHOGANY 11.5- titanium dioxide gel
BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 BAMBOO 5.5- titanium dioxide gel
BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 BIRCH 1.5- titanium dioxide gel
BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 DESERT 6.5- titanium dioxide gel
BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 DUNE 7.5- titanium dioxide gel
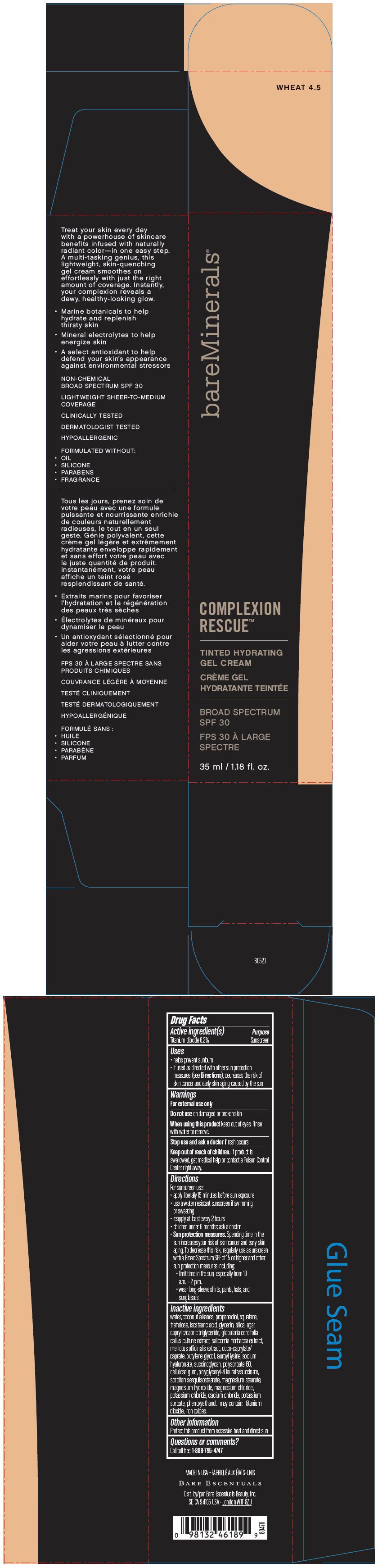
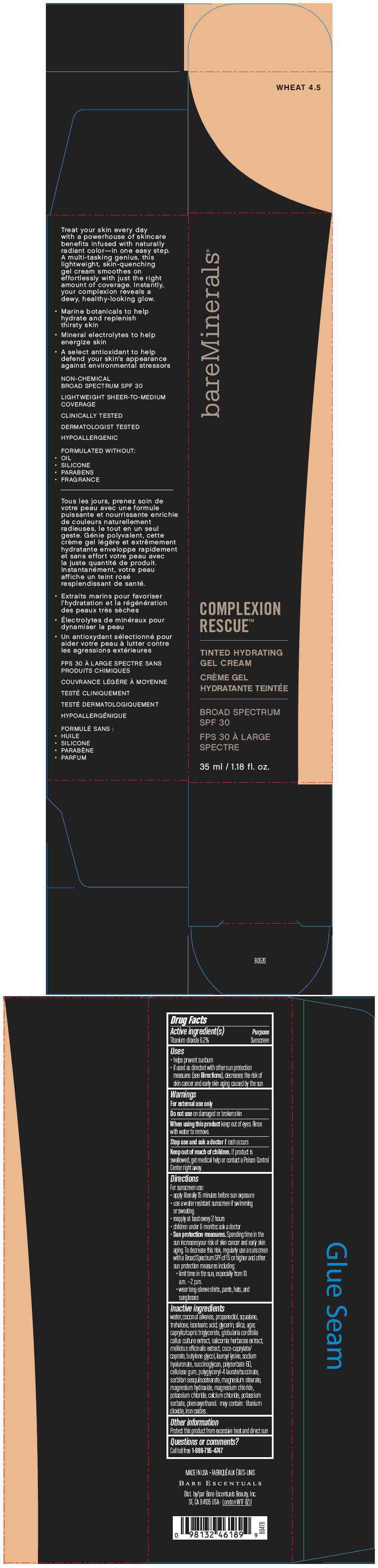
BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 WHEAT 4.5- titanium dioxide gel
BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 TERRA 8.5- titanium dioxide gel
-
NDC Code(s):
98132-274-10,
98132-275-10,
98132-276-10,
98132-277-10, view more98132-278-10, 98132-279-10, 98132-280-10, 98132-281-10, 98132-282-10, 98132-283-10
- Packager: Orveon Global US LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient(s)
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: ask a doctor
-
Sun protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
-
Inactive ingredients
water, coconut alkanes, propanediol, squalane, trehalose, isostearic acid, glycerin, silica, agar, caprylic/capric triglyceride, globularia cordifolia callus culture extract, salicornia herbacea extract, melilotus officinalis extract, coco-caprylate/ caprate, butylene glycol, lauroyl lysine, sodium hyaluronate, succinoglycan, polysorbate 60, cellulose gum, polyglyceryl-4 laurate/succinate, sorbitan sesquiisostearate, magnesium stearate, magnesium hydroxide, magnesium chloride, potassium chloride, calcium chloride, potassium sorbate, phenoxyethanol. may contain: titanium dioxide, iron oxides.
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Box - Cashew 3.5
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Box - Cinnamon 10.5
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Box - Cedar 11
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Box - Mahogany 11.5
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Box - Bamboo 5.5
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Box - Birch 1.5
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Box - Desert 6.5
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Box - Dune 7.5
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Box - Wheat 4.5
- PRINCIPAL DISPLAY PANEL - 35 ml Bottle Box - Terra 8.5
-
INGREDIENTS AND APPEARANCE
BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 CASHEW 3.5
titanium dioxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-274 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 62 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) PROPANEDIOL (UNII: 5965N8W85T) SQUALANE (UNII: GW89575KF9) TREHALOSE (UNII: B8WCK70T7I) ISOSTEARIC ACID (UNII: X33R8U0062) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) AGAR, UNSPECIFIED (UNII: 89T13OHQ2B) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SALICORNIA EUROPAEA (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAUROYL LYSINE (UNII: 113171Q70B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYSORBATE 60 (UNII: CAL22UVI4M) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) MAGNESIUM STEARATE (UNII: 70097M6I30) MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-274-10 1 in 1 BOX 12/01/2019 1 35 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2019 BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 CINNAMON 10.5
titanium dioxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-275 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 62 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) PROPANEDIOL (UNII: 5965N8W85T) SQUALANE (UNII: GW89575KF9) TREHALOSE (UNII: B8WCK70T7I) ISOSTEARIC ACID (UNII: X33R8U0062) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) AGAR, UNSPECIFIED (UNII: 89T13OHQ2B) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SALICORNIA EUROPAEA (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAUROYL LYSINE (UNII: 113171Q70B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYSORBATE 60 (UNII: CAL22UVI4M) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) MAGNESIUM STEARATE (UNII: 70097M6I30) MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-275-10 1 in 1 BOX 12/01/2019 1 35 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2019 BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 CEDAR 11
titanium dioxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-276 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 62 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) PROPANEDIOL (UNII: 5965N8W85T) SQUALANE (UNII: GW89575KF9) TREHALOSE (UNII: B8WCK70T7I) ISOSTEARIC ACID (UNII: X33R8U0062) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) AGAR, UNSPECIFIED (UNII: 89T13OHQ2B) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SALICORNIA EUROPAEA (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAUROYL LYSINE (UNII: 113171Q70B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYSORBATE 60 (UNII: CAL22UVI4M) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) MAGNESIUM STEARATE (UNII: 70097M6I30) MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-276-10 1 in 1 BOX 12/01/2019 1 35 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2019 BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 MAHOGANY 11.5
titanium dioxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-277 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 62 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) PROPANEDIOL (UNII: 5965N8W85T) SQUALANE (UNII: GW89575KF9) TREHALOSE (UNII: B8WCK70T7I) ISOSTEARIC ACID (UNII: X33R8U0062) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) AGAR, UNSPECIFIED (UNII: 89T13OHQ2B) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SALICORNIA EUROPAEA (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAUROYL LYSINE (UNII: 113171Q70B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYSORBATE 60 (UNII: CAL22UVI4M) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) MAGNESIUM STEARATE (UNII: 70097M6I30) MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-277-10 1 in 1 BOX 12/01/2019 1 35 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2019 BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 BAMBOO 5.5
titanium dioxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-278 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 62 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) PROPANEDIOL (UNII: 5965N8W85T) SQUALANE (UNII: GW89575KF9) TREHALOSE (UNII: B8WCK70T7I) ISOSTEARIC ACID (UNII: X33R8U0062) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) AGAR, UNSPECIFIED (UNII: 89T13OHQ2B) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SALICORNIA EUROPAEA (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAUROYL LYSINE (UNII: 113171Q70B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYSORBATE 60 (UNII: CAL22UVI4M) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) MAGNESIUM STEARATE (UNII: 70097M6I30) MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-278-10 1 in 1 BOX 12/01/2019 1 35 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2019 BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 BIRCH 1.5
titanium dioxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-279 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 62 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) PROPANEDIOL (UNII: 5965N8W85T) SQUALANE (UNII: GW89575KF9) TREHALOSE (UNII: B8WCK70T7I) ISOSTEARIC ACID (UNII: X33R8U0062) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) AGAR, UNSPECIFIED (UNII: 89T13OHQ2B) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SALICORNIA EUROPAEA (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAUROYL LYSINE (UNII: 113171Q70B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYSORBATE 60 (UNII: CAL22UVI4M) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) MAGNESIUM STEARATE (UNII: 70097M6I30) MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-279-10 1 in 1 BOX 12/01/2019 1 35 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2019 BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 DESERT 6.5
titanium dioxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-280 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 62 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYSORBATE 60 (UNII: CAL22UVI4M) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) MAGNESIUM STEARATE (UNII: 70097M6I30) MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) PROPANEDIOL (UNII: 5965N8W85T) SQUALANE (UNII: GW89575KF9) TREHALOSE (UNII: B8WCK70T7I) ISOSTEARIC ACID (UNII: X33R8U0062) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) AGAR, UNSPECIFIED (UNII: 89T13OHQ2B) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SALICORNIA EUROPAEA (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAUROYL LYSINE (UNII: 113171Q70B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-280-10 1 in 1 BOX 12/01/2019 1 35 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2019 BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 DUNE 7.5
titanium dioxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-281 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 62 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) PROPANEDIOL (UNII: 5965N8W85T) SQUALANE (UNII: GW89575KF9) TREHALOSE (UNII: B8WCK70T7I) ISOSTEARIC ACID (UNII: X33R8U0062) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) AGAR, UNSPECIFIED (UNII: 89T13OHQ2B) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SALICORNIA EUROPAEA (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAUROYL LYSINE (UNII: 113171Q70B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYSORBATE 60 (UNII: CAL22UVI4M) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) MAGNESIUM STEARATE (UNII: 70097M6I30) MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-281-10 1 in 1 BOX 12/01/2019 1 35 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2019 BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 WHEAT 4.5
titanium dioxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-282 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 62 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) PROPANEDIOL (UNII: 5965N8W85T) SQUALANE (UNII: GW89575KF9) TREHALOSE (UNII: B8WCK70T7I) ISOSTEARIC ACID (UNII: X33R8U0062) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) AGAR, UNSPECIFIED (UNII: 89T13OHQ2B) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SALICORNIA EUROPAEA (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAUROYL LYSINE (UNII: 113171Q70B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYSORBATE 60 (UNII: CAL22UVI4M) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) MAGNESIUM STEARATE (UNII: 70097M6I30) MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-282-10 1 in 1 BOX 12/01/2019 1 35 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2019 BAREMINERALS COMPLEXION RESCUE TINTED HYDRATING BROAD SPECTRUM SPF 30 TERRA 8.5
titanium dioxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-283 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 62 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) PROPANEDIOL (UNII: 5965N8W85T) SQUALANE (UNII: GW89575KF9) TREHALOSE (UNII: B8WCK70T7I) ISOSTEARIC ACID (UNII: X33R8U0062) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) AGAR, UNSPECIFIED (UNII: 89T13OHQ2B) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SALICORNIA EUROPAEA (UNII: 6ADL50JAKW) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAUROYL LYSINE (UNII: 113171Q70B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYSORBATE 60 (UNII: CAL22UVI4M) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) MAGNESIUM STEARATE (UNII: 70097M6I30) MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-283-10 1 in 1 BOX 12/01/2019 1 35 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2019 Labeler - Orveon Global US LLC (118344494)