Label: RAMIPRIL capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 51655-301-30 - Packager: Northwind Pharmaceuticals
- This is a repackaged label.
- Source NDC Code(s): 16252-573
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 7, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
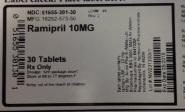
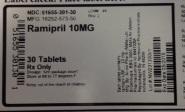
NDC: 51655-301-30
MFG: 16252-573-50
Ramipril 10MG
30 Tablets
Rx Only
Dosage: See package insert
Store at 60 to 77 degrees F.
Keep out of reach of children.
Each tablet contains: ramipril, USP 10mg
Mfg by: Arrow Pharm (Malta) Ltd Birzebbugia, BBG 3000 Malta
for Watson Pharmaceuticals Lot # 844703M
Repackaged by Northwind Pharmaceuticals
Indianapolis, IN 46256
Lot# NW23130001
Exp Date: 05/2015
- WARNINGS AND PRECAUTIONS
-
INGREDIENTS AND APPEARANCE
RAMIPRIL
ramipril capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51655-301(NDC:16252-573) Route of Administration oral Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RAMIPRIL (UNII: L35JN3I7SJ) (RAMIPRILAT - UNII:6N5U4QFC3G) RAMIPRIL 10 mg in 30 Product Characteristics Color white, blue Score no score Shape capsule Size 14mm Flavor Imprint Code RP10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51655-301-30 30 in 1 BOTTLE, DISPENSING Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076549 05/07/2014 Labeler - Northwind Pharmaceuticals (036986393) Registrant - Northwind Pharmaceuticals (036986393) Establishment Name Address ID/FEI Business Operations Northwind Pharmaceuticals 036986393 repack(51655-301)