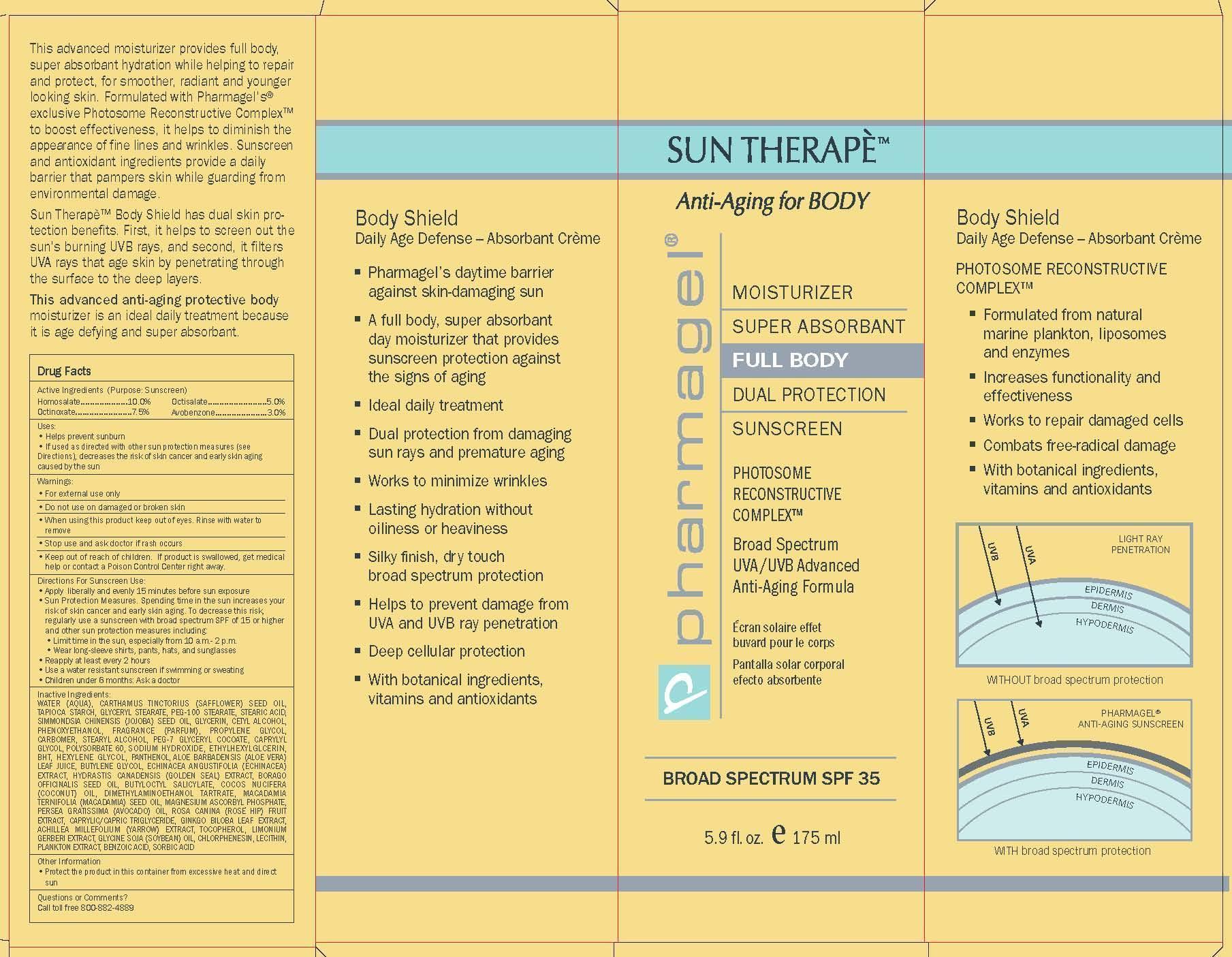
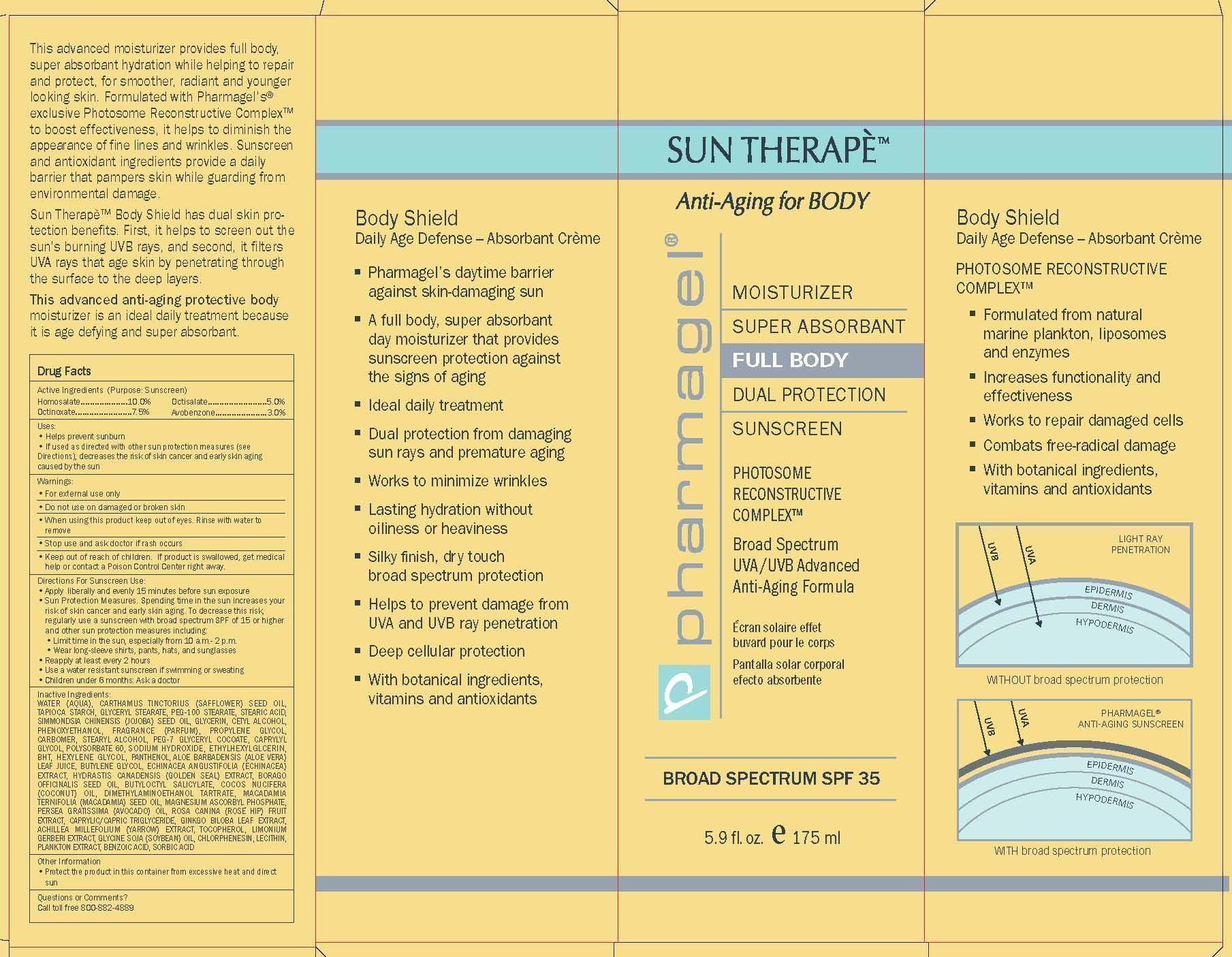
Label: SUN THERAPE BODY SPF-35- homosalate, octinoxate, octisalate, avobenzone lotion
- NDC Code(s): 67879-303-11, 67879-303-51
- Packager: PHARMAGEL INTERNATIONAL INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- OTHER SAFETY INFORMATION
-
Directions For Sunscreen Use:
• Apply liberally and evenly 15 minutes before sun exposure
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including:
• Limit time in the sun, especially from 10 a.m.- 2 p.m.
• Wear long-sleeve shirts, pants, hats, and sunglasses
• Reapply at least every 2 hours
• Use a water resistant sunscreen if swimming or sweating
• Children under 6 months: Ask a doctor -
INACTIVE INGREDIENTS:
WATER (AQUA), CARTHAMUS TINCTORIUS (SAFFLOWER) SEED OIL, TAPIOCA
STARCH, GLYCERYL STEARATE, PEG-100 STEARATE, STEARIC ACID, SIMMONDSIA
CHINENSIS (JOJOBA) SEED OIL, GLYCERIN, CETYL ALCOHOL, PHENOXYETHANOL,
FRAGRANCE (PARFUM), PROPYLENE GLYCOL, CARBOMER, STEARYL ALCOHOL,
PEG-7 GLYCERYL COCOATE, CAPRYLYL GLYCOL, POLYSORBATE 60, SODIUM
HYDROXIDE, ETHYLHEXYLGLCERIN, BHT, HEXYLENE GLYCOL, PANTHENOL, ALOE
BARBADENSIS (ALOE VERA) LEAF JUICE, BUTYLENE GLYCOL, ECHINACEA
ANGUSTIFOLIA (ECHINACEA) EXTRACT, HYDRASTIS CANADENSIS (GOLDEN
SEAL) EXTRACT, BORAGO OFFICINALIS SEED OIL, BUTYLOCTYL SALICYLATE,
COCOS NUCIFERA (COCONUT) OIL, DIMETHYLAMINOETHANOL TARTRATE,
MACADAMIA TERNIFOLIA (MACADAMIA) SEED OIL, MAGNESIUM ASCORBYL
PHOSPHATE, PERSEA GRATISSIMA (AVOCADO) OIL, ROSA CANINA (ROSE HIP)
FRUIT EXTRACT, CAPRYLIC/CAPRIC TRIGLYCERIDE, GINKGO BILOBA LEAF EXTRACT,
ACHILLEA MILLEFOLIUM (YARROW) EXTRACT, TOCOPHEROL, LIMONIUM GERBERI
EXTRACT, GLYCINE SOJA (SOYBEAN) OIL, CHLORPHENESIN, LECITHIN, PLANKTON
EXTRACT, BENZOIC ACID, SORBIC ACID
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUN THERAPE BODY SPF-35
homosalate, octinoxate, octisalate, avobenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67879-303 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SAFFLOWER OIL (UNII: 65UEH262IS) STARCH, TAPIOCA (UNII: 24SC3U704I) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) STEARIC ACID (UNII: 4ELV7Z65AP) JOJOBA OIL (UNII: 724GKU717M) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POLYSORBATE 60 (UNII: CAL22UVI4M) SODIUM HYDROXIDE (UNII: 55X04QC32I) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) HEXYLENE GLYCOL (UNII: KEH0A3F75J) PANTHENOL (UNII: WV9CM0O67Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) GOLDENSEAL (UNII: ZW3Z11D0JV) BORAGE OIL (UNII: F8XAG1755S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) COCONUT OIL (UNII: Q9L0O73W7L) DEANOL BITARTRATE (UNII: D240J05W14) MACADAMIA OIL (UNII: 515610SU8C) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) AVOCADO OIL (UNII: 6VNO72PFC1) ROSA CANINA FRUIT (UNII: 3TNW8D08V3) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GINKGO (UNII: 19FUJ2C58T) ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) TOCOPHEROL (UNII: R0ZB2556P8) LIMONIUM GERBERI WHOLE (UNII: 2J5K7YCF9F) SOYBEAN OIL (UNII: 241ATL177A) CHLORPHENESIN (UNII: I670DAL4SZ) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) CHONDRUS CRISPUS (UNII: OQS23HUA1X) BENZOIC ACID (UNII: 8SKN0B0MIM) SORBIC ACID (UNII: X045WJ989B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67879-303-51 1 in 1 BOX 06/18/2015 1 NDC:67879-303-11 175 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/18/2015 Labeler - PHARMAGEL INTERNATIONAL INC (603215182)