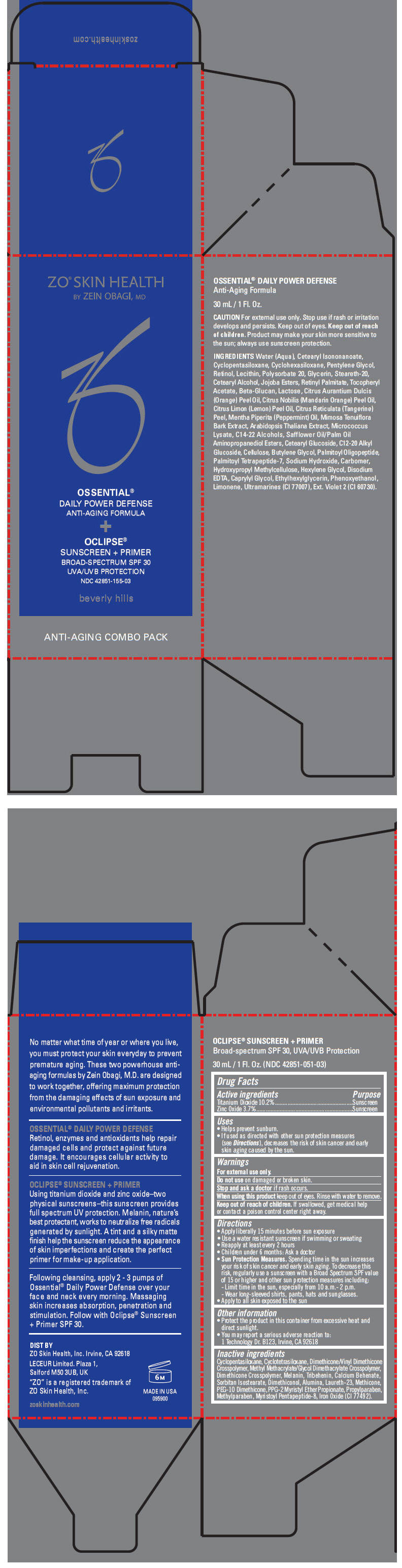
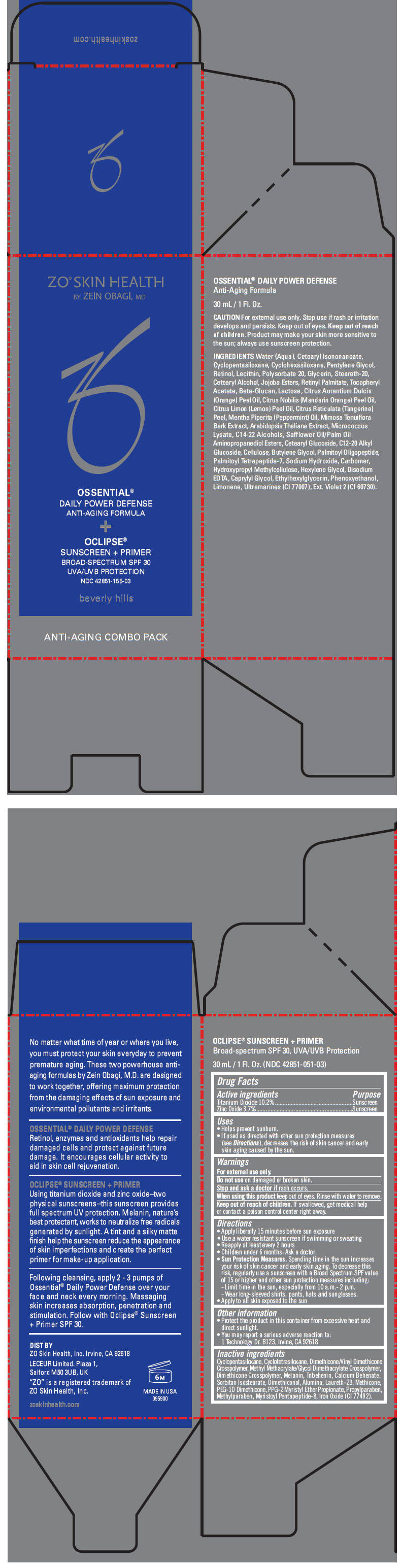
Label: ZO SKIN HEALTH OSSENTIAL DAILY POWER DEFENSE ANTI-AGING FORMULA AND OCLIPSE SUNSCREEN PLUS PRIMER BROAD SPECTRUM SPF 30 UVA/UVB PROTECTION- titanium dioxide and zinc oxide kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 42851-051-03, 42851-155-03 - Packager: ZO Skin Health, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 22, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- -
- Limit time in the sun, especially from 10 a.m.- 2 p.m.
- -
- Wear long-sleeved shirts, pants, hats and sunglasses.
- Apply to all skin exposed to the sun
- Other information
-
Inactive ingredients
Cyclopentasiloxane, Cyclotetrasiloxane, Dimethicone/Vinyl Dimethicone Crosspolymer, Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer, Dimethicone Crosspolymer, Melanin, Tribehenin, Calcium Behenate, Sorbitan Isostearate, Dimethiconol, Alumina, Laureth-23, Methicone, PEG-10 Dimethicone, PPG-2 Myristyl Ether Propionate, Propylparaben, Methylparaben, Myristoyl Pentapeptide-8, Iron Oxide (CI 77492).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
ZO SKIN HEALTH OSSENTIAL DAILY POWER DEFENSE ANTI-AGING FORMULA AND OCLIPSE SUNSCREEN PLUS PRIMER BROAD SPECTRUM SPF 30 UVA/UVB PROTECTION
titanium dioxide and zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42851-155 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-155-03 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PUMP 30 mL Part 2 1 BOTTLE, PUMP 30 mL Part 1 of 2 OCLIPSE SUNSCREEN PLUS PRIMER BROAD-SPECTRUM SPF 30 UVA/UVB PROTECTION
titanium dioxide and zinc oxide lotionProduct Information Item Code (Source) NDC:42851-051 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) TITANIUM DIOXIDE 102 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) ZINC OXIDE 37 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 4 (UNII: CZ227117JE) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) TRIBEHENIN (UNII: 8OC9U7TQZ0) CALCIUM BEHENATE (UNII: J5VFA9V6YG) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) DIMETHICONOL (40 CST) (UNII: 343C7U75XW) ALUMINUM OXIDE (UNII: LMI26O6933) LAURETH-23 (UNII: N72LMW566G) METHICONE (20 CST) (UNII: 6777U11MKT) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PPG-2 MYRISTYL ETHER PROPIONATE (UNII: 88R97D8U8A) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-051-03 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/01/2012 Part 2 of 2 OSSENTIAL DAILY POWER DEFENSE ANTI-AGING FORMULA
cleansing (cold creams, cleansing lotions, liquids, and pads) lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR CETEARYL ISONONANOATE (UNII: P5O01U99NI) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR CYCLOMETHICONE 6 (UNII: XHK3U310BA) INGR PENTYLENE GLYCOL (UNII: 50C1307PZG) INGR RETINOL (UNII: G2SH0XKK91) INGR EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR STEARETH-20 (UNII: L0Q8IK9E08) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR JOJOBA BUTTER (UNII: XIA46H803R) INGR VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR CURDLAN (UNII: 6930DL209R) INGR LACTOSE (UNII: J2B2A4N98G) INGR ORANGE OIL (UNII: AKN3KSD11B) INGR MANDARIN OIL (UNII: NJO720F72R) INGR LEMON OIL (UNII: I9GRO824LL) INGR PEPPERMINT OIL (UNII: AV092KU4JH) INGR MIMOSA TENUIFLORA BARK (UNII: 515MQE449I) INGR ARABIDOPSIS THALIANA (UNII: AI3L60HQ81) INGR C14-22 ALCOHOLS (UNII: B1K89384RJ) INGR CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) INGR C12-20 ALKYL GLUCOSIDE (UNII: K67N5Z1RUA) INGR POWDERED CELLULOSE (UNII: SMD1X3XO9M) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR PALMITOYL OLIGOPEPTIDE (UNII: HO4ZT5S86C) INGR PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) INGR HYPROMELLOSES (UNII: 3NXW29V3WO) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR LIMONENE, (+/-)- (UNII: 9MC3I34447) INGR ULTRAMARINE BLUE (UNII: I39WR998BI) INGR EXT. D&C VIOLET NO. 2 (UNII: G5UX3K0728) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 01/01/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 01/01/2012 Labeler - ZO Skin Health, Inc. (826468527) Establishment Name Address ID/FEI Business Operations Owen Biosciences 790003045 MANUFACTURE(42851-155) Establishment Name Address ID/FEI Business Operations PakLab, Inc. 177711082 MANUFACTURE(42851-155)