Label: GAVISCON- aluminum hydroxide and magnesium carbonate liquid
- NDC Code(s): 0135-0094-41, 0135-0094-42, 0135-0095-41, 0135-0574-01
- Packager: Haleon US Holdings LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 15 mL tablespoonful) Regular Strength
- Active ingredients (in each 5 mL teaspoonful) Extra Strength
- Purpose
- Uses
-
Warnings
Ask a doctor or pharmacist before use if you
- have kidney disease.
- are on a sodium-restricted diet or a magnesium-restricted diet.
- are taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product (Regular Strength)
- do not take more than 8 tablespoonfuls in 24 hours
- do not use the maximum dosage for more than 2 weeks except under the advice and supervision of a doctor
- laxative effect may occur
- Directions (Regular Strength)
- Directions (Extra Strength)
- Other information (Regular Strength)
- Other information (Extra Strength)
- Inactive ingredients (Regular Strength)
- Inactive ingredients (Extra Strength Cool Mint)
- Inactive Ingredients (Extra Strength Cherry)
- Questions or comments?
- Additional Information
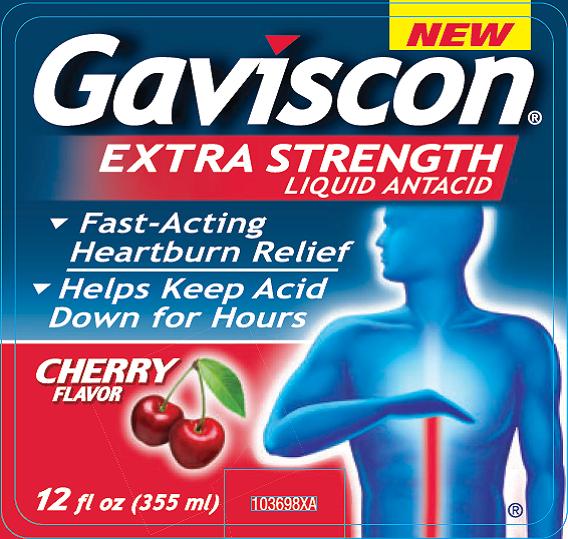
- Principal Display Panel
- Principal Display Panel
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GAVISCON
aluminum hydroxide and magnesium carbonate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0135-0094 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 95 mg in 15 mL MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 358 mg in 15 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GLYCERIN (UNII: PDC6A3C0OX) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM ALGINATE (UNII: C269C4G2ZQ) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color green Score Shape Size Flavor MINT (cool mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0135-0094-41 355 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/14/2011 2 NDC:0135-0094-42 177 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/14/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 01/14/2011 GAVISCON
aluminum hydroxide and magnesium carbonate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0135-0095 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 254 mg in 5 mL MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 237.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) SACCHARIN SODIUM (UNII: SB8ZUX40TY) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM ALGINATE (UNII: C269C4G2ZQ) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color green Score Shape Size Flavor MINT (cool mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0135-0095-41 355 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/14/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 01/14/2011 GAVISCON
aluminum hydroxide and magnesium carbonate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0135-0574 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 254 mg in 5 mL MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 237.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) SACCHARIN SODIUM (UNII: SB8ZUX40TY) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM ALGINATE (UNII: C269C4G2ZQ) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color white Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0135-0574-01 355 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 08/01/2014 Labeler - Haleon US Holdings LLC (079944263)