Label: TEETH DESENSITIZING AND REMINERALIZING GEL- potassium nitrate,sodium fluoride,sodium monofluorophosphate paste
-
Contains inactivated NDC Code(s)
NDC Code(s): 49410-200-01, 49410-200-02, 49410-200-03, 49410-200-04, view more49410-200-05, 49410-200-06, 49410-200-07, 49410-200-08 - Packager: Fuzhou Difeng Bio-tech Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 6, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
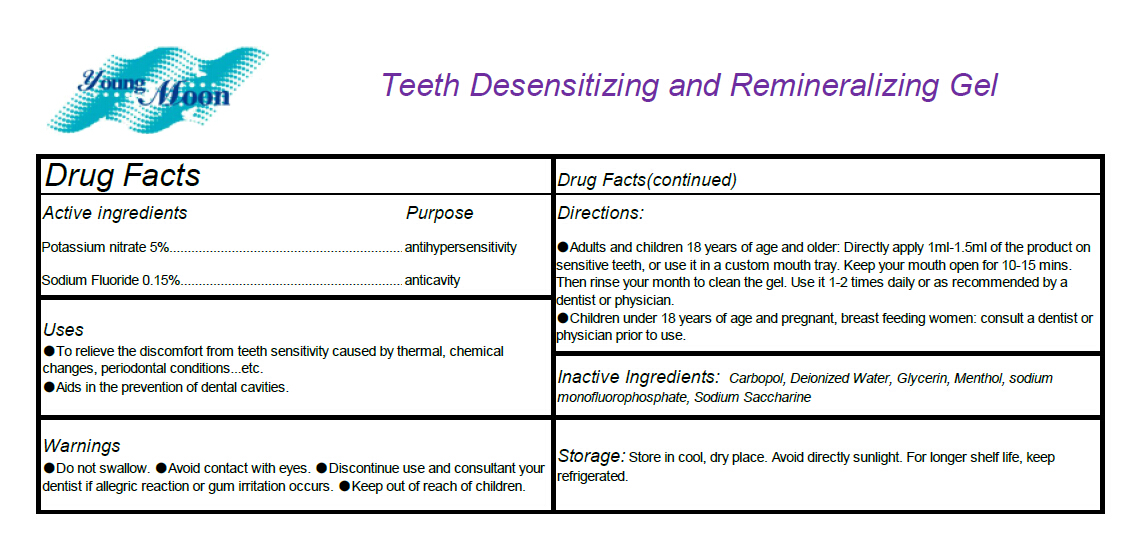
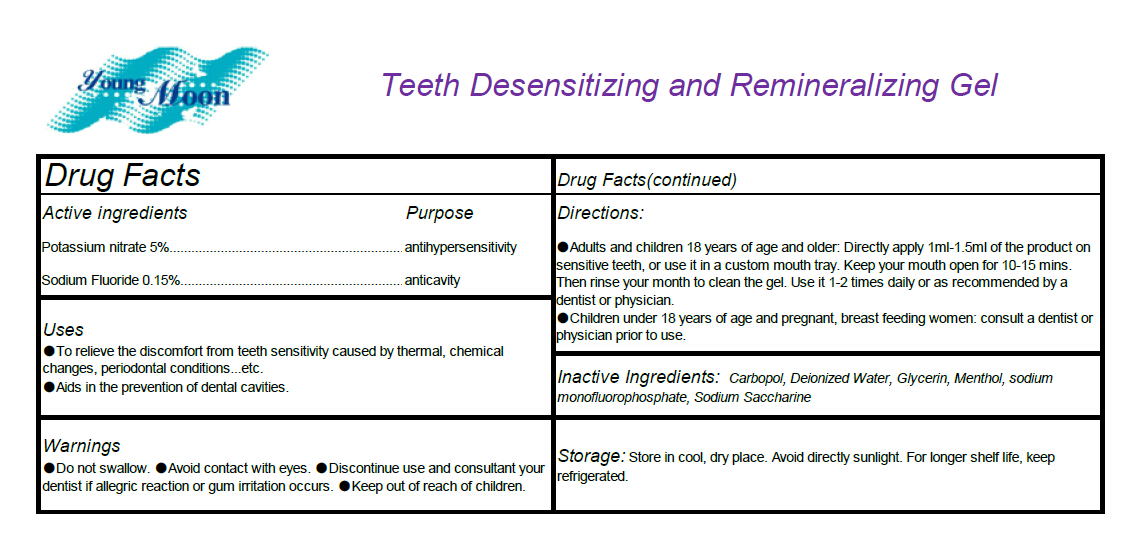
- Active ingredients
- Uses
- Warnings
-
Directions
Adults and children 18 years of age and older: Directly apply 1ml-1.5ml of the product on
sensitive teeth, or use it in a custom mouth tray. Keep your mouth open for 10-15 mins. Then rinse
your month to clean the gel. Use it 1-2 times daily or as recommended by a dentist or physician.
Children under 18 years of age and pregnant, breast feeding women: consult a dentist or
physician prior to use. - INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TEETH DESENSITIZING AND REMINERALIZING GEL
potassium nitrate,sodium fluoride,sodium monofluorophosphate pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49410-200 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM NITRATE (UNII: RU45X2JN0Z) (NITRATE ION - UNII:T93E9Y2844) POTASSIUM NITRATE 50 mg in 1 g SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) MENTHOL (UNII: L7T10EIP3A) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) CARBOMER HOMOPOLYMER TYPE A (UNII: F68VH75CJC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49410-200-01 5 in 1 CARTON 01/08/2018 1 200 in 1 CONTAINER 1 1.5 g in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC:49410-200-02 5 in 1 CARTON 01/08/2018 2 100 in 1 CONTAINER 2 2 g in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 3 NDC:49410-200-03 5 in 1 CARTON 01/08/2018 3 100 in 1 CONTAINER 3 4 g in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 4 NDC:49410-200-04 5 in 1 CARTON 01/08/2018 4 80 in 1 CONTAINER 4 6 g in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 5 NDC:49410-200-05 6 in 1 CARTON 01/08/2018 5 300 in 1 CONTAINER 5 4.5 g in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 6 NDC:49410-200-06 6 in 1 CARTON 01/08/2018 6 200 in 1 CONTAINER 6 10 g in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 7 NDC:49410-200-07 6 in 1 CARTON 01/08/2018 7 333 in 1 CONTAINER 7 3 g in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 8 NDC:49410-200-08 300 in 1 CARTON 01/08/2018 8 3.5 g in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 03/07/2016 Labeler - Fuzhou Difeng Bio-tech Co., Ltd. (528195950) Registrant - Fuzhou Difeng Bio-tech Co., Ltd. (528195950) Establishment Name Address ID/FEI Business Operations Fuzhou Difeng Bio-tech Co., Ltd. 528195950 manufacture(49410-200)