Label: BISOPROLOL FUMARATE tablet, film coated
- NDC Code(s): 70771-1726-1, 70771-1726-3, 70771-1727-1, 70771-1727-3
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated July 26, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
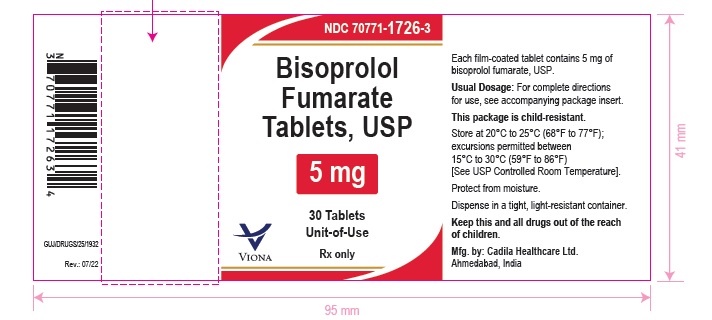
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BISOPROLOL FUMARATE
bisoprolol fumarate tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1726 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISOPROLOL FUMARATE (UNII: UR59KN573L) (BISOPROLOL - UNII:Y41JS2NL6U) BISOPROLOL FUMARATE 5 mg Inactive Ingredients Ingredient Name Strength ALUMINUM OXIDE (UNII: LMI26O6933) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) CROSPOVIDONE (12 MPA.S AT 5%) (UNII: 40UAA97IT9) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) FD&C RED NO. 40 (UNII: WZB9127XOA) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK (light pink to pink) Score 2 pieces Shape ROUND Size 6mm Flavor Imprint Code 111 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1726-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/15/2022 2 NDC:70771-1726-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/15/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215680 09/15/2022 BISOPROLOL FUMARATE
bisoprolol fumarate tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1727 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISOPROLOL FUMARATE (UNII: UR59KN573L) (BISOPROLOL - UNII:Y41JS2NL6U) BISOPROLOL FUMARATE 10 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) CROSPOVIDONE (12 MPA.S AT 5%) (UNII: 40UAA97IT9) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (white to off white colored with occasional greyish) Score no score Shape ROUND Size 7mm Flavor Imprint Code 112 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1727-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/15/2022 2 NDC:70771-1727-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/15/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215680 09/15/2022 Labeler - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 863362789 ANALYSIS(70771-1726, 70771-1727) , MANUFACTURE(70771-1726, 70771-1727)