Label: NO MORE PRODUCTS- glycerin, kaolin, calamine, menthol cream
- NDC Code(s): 71831-005-01, 71831-005-02, 71831-005-03
- Packager: PRANICURA GROUP LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
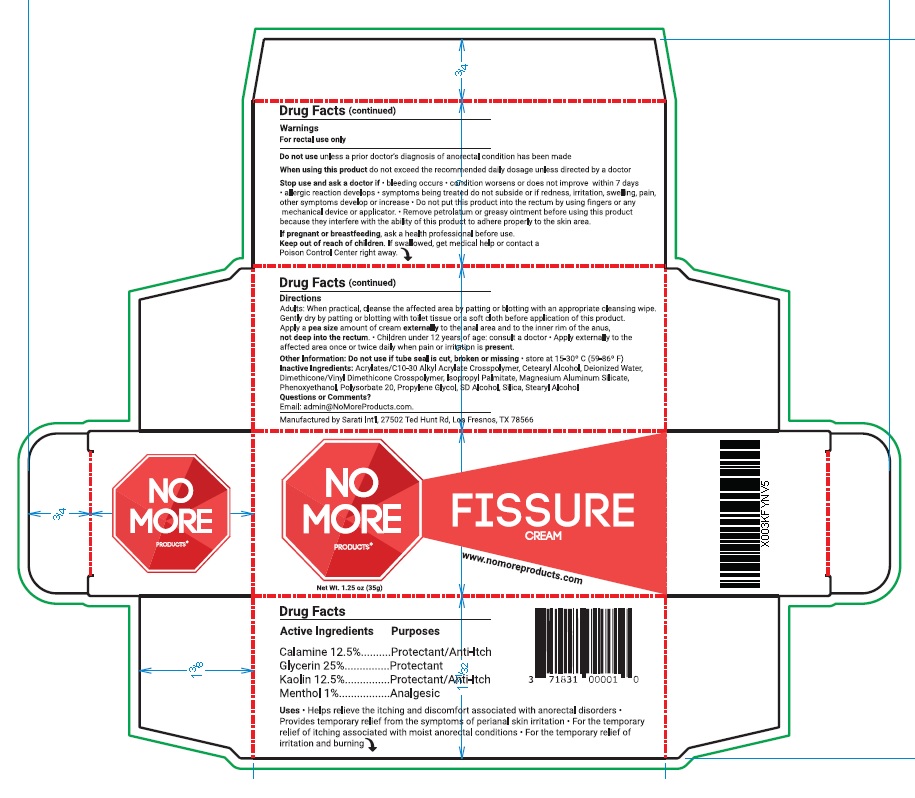
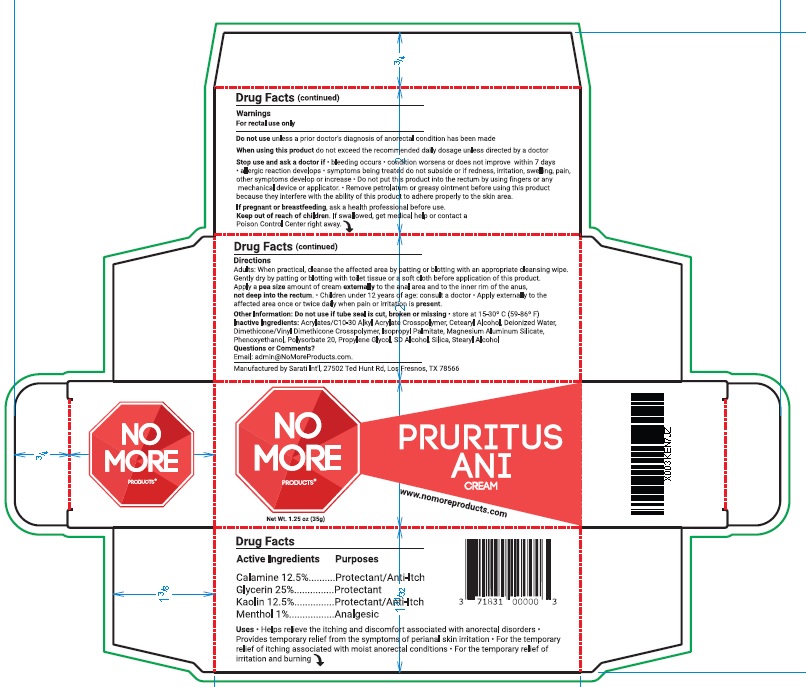
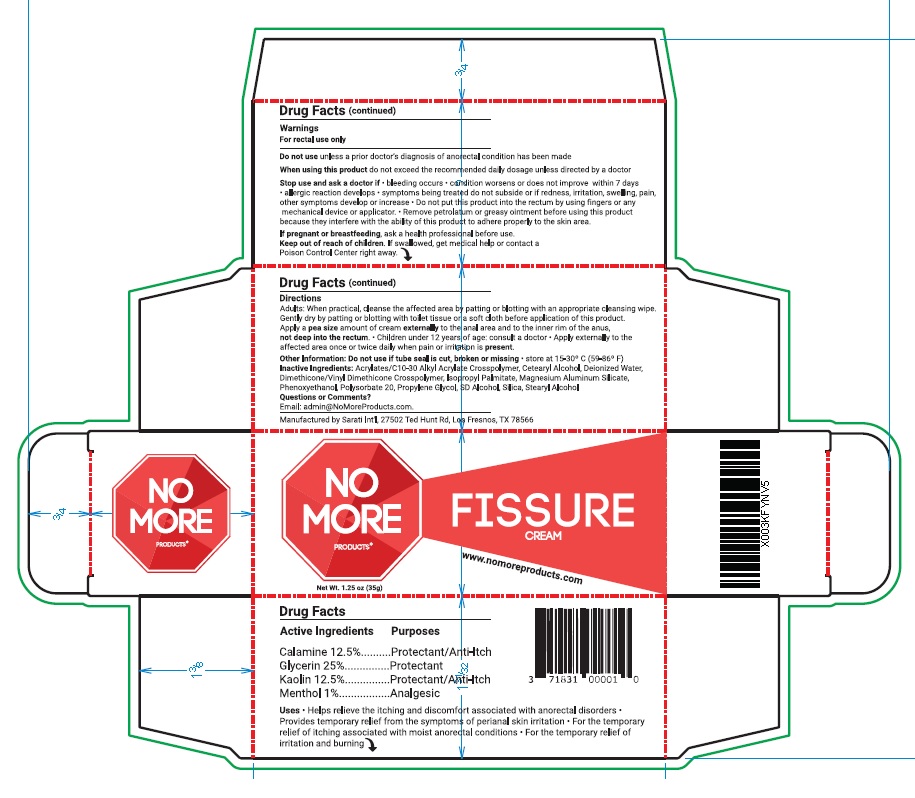
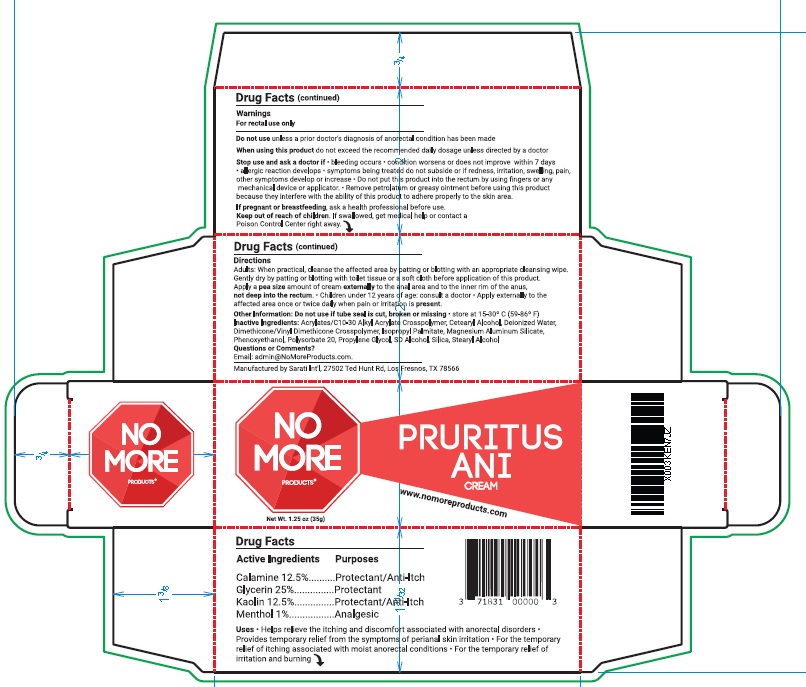
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For rectal use only
Do not use unless a prior doctor’s diagnosis of anorectal condition has been made
When using this product do not exceed the recommended daily dosage unless directed by a doctor
Stop use and ask a doctor if • bleeding occurs • condition worsens or does not improve within 7 days
• allergic reaction develops • symptoms being treated do not subside or if redness, irritation, swelling, pain,
other symptoms develop or increase • Do not put this product into the rectum by using fingers or any
mechanical device or applicator. • Remove petrolatum or greasy ointment before using this product
because they interfere with the ability of this product to adhere properly to the skin area.
If pregnant or breastfeeding, ask a health professional before use - KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Adults: When practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe.
Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product.
Apply a pea size amount of cream externally to the anal area and to the inner rim of the anus,
not deep into the rectum. • Children under 12 years of age: consult a doctor • Apply externally to the
affected area once or twice daily when pain or irritation is present. - OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- No More Product Labeling
-
INGREDIENTS AND APPEARANCE
NO MORE PRODUCTS
glycerin, kaolin, calamine, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71831-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 25.0 g in 100 g KAOLIN (UNII: 24H4NWX5CO) (KAOLIN - UNII:24H4NWX5CO) KAOLIN 12.5 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 12.5 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1.0 g in 100 g Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) WATER (UNII: 059QF0KO0R) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALCOHOL (UNII: 3K9958V90M) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71831-005-03 1 in 1 BOX 03/01/2023 1 35 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:71831-005-02 1 in 1 BOX 03/01/2023 2 35 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:71831-005-01 1 in 1 BOX 03/01/2023 3 35 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 03/01/2023 Labeler - PRANICURA GROUP LLC (118320102)