Label: NORETHINDRONE ACETATE AND ETHINYL ESTRADIOL tablet
-
NDC Code(s):
75834-129-29,
75834-129-84,
75834-129-90,
75834-130-29, view more75834-130-84, 75834-130-90
- Packager: Nivagen Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 24, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NORETHINDRONE ACETATE and ETHINYL ESTRADIOL TABLETS safely and effectively. See full prescribing information for NORETHINDRONE ACETATE and ETHINYL ESTRADIOL TABLETS.
Norethindrone Acetate and Ethinyl Estradiol Tablets USP, for Oral Use
Initial U.S. Approval: 1968WARNING: CARDIOVASCULAR DISORDERS, BREAST CANCER, ENDOMETRIAL CANCER AND PROBABLE DEMENTIA
See full prescribing information for complete boxed warning.
Estrogen Plus Progestin Therapy
- Estrogen plus progestin therapy should not be used for the prevention of cardiovascular disease or dementia (5.1, 5.3)
- The Women's Health Initiative (WHI) estrogen plus progestin substudy reported increased risks of stroke, deep vein thrombosis (DVT), pulmonary embolism (PE), and myocardial infarction (MI) (5.1)
- The WHI estrogen plus progestin substudy reported an increased risk of invasive breast cancer (5.2)
- The WHI Memory Study (WHIMS) estrogen plus progestin ancillary study of WHI reported an increased risk of probable dementia in postmenopausal women 65 years of age and older (5.3)
Estrogen-Alone Therapy
- There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens (5.2)
- Estrogen-alone therapy should not be used for the prevention of cardiovascular disease or dementia (5.1, 5.3)
- The WHI estrogen-alone substudy reported increased risks of stroke and DVT (5.1)
- The WHIMS estrogen-alone ancillary study of WHI reported an increased risk of probable dementia in postmenopausal women 65 years of age and older (5.3)
RECENT MAJOR CHANGES
Warnings and Precautions, Malignant Neoplasms (5.2) 11/2017
INDICATIONS AND USAGE
Norethindrone acetate and Ethinyl estradiol tablets are an estrogen plus progestin indicated in a woman with a uterus for:
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- Undiagnosed abnormal genital bleeding (4)
- Known, suspected, or history of breast cancer (4, 5.2)
- Known or suspected estrogen-dependent neoplasia (4, 5.2)
- Active DVT, PE, or history of these conditions (4, 5.1)
- Active arterial thromboembolic disease (for example, stroke and MI), or a history of these conditions (4, 5.1)
- Known anaphylactic reaction or angioedema to norethindrone acetate and ethinyl estradiol tablets. (4)
- Known liver impairment or disease (4, 5.10)
- Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders (4)
- Known or suspected pregnancy (4, 8.1)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions (incidence greater than or equal to 5 percent) are headache, abdominal pain, breast pain, and edema (generalized). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Nivagen Pharmaceuticals Toll-free at 1-877-977-0687 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Nursing Mothers: Estrogen administration to nursing women has been shown to decrease the quantity and quality of breast milk (8.3)
- Geriatric Use: An increased risk of probable dementia in women over 65 years of age was reported in the Women's Health Initiative Memory ancillary studies of the Women's Health Initiative (5.3, 8.5)
See 17 for PATIENT COUNSELING INFORMATION, FDA-approved patient labeling and FDA-approved patient labeling.
Revised: 8/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CARDIOVASCULAR DISORDERS, BREAST CANCER, ENDOMETRIAL CANCER AND PROBABLE DEMENTIA
1 INDICATIONS AND USAGE
1.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause
1.2 Prevention of Postmenopausal Osteoporosis
2 DOSAGE AND ADMINISTRATION
2.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause
2.2 Prevention of Postmenopausal Osteoporosis
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Disorders
5.2 Malignant Neoplasms
5.3 Probable Dementia
5.4 Gallbladder Disease
5.5 Hypercalcemia
5.6 Visual Abnormalities
5.7 Addition of a Progestin When a Woman Has Not Had a Hysterectomy
5.8 Elevated Blood Pressure
5.9 Hypertriglyceridemia
5.10 Hepatic Impairment and/or a Past History of Cholestatic Jaundice
5.11 Hypothyroidism
5.12 Fluid Retention
5.13 Hypocalcemia
5.14 Exacerbation of Endometriosis
5.15 Hereditary Angioedema
5.16 Exacerbation of Other Conditions
5.17 Laboratory Tests
5.18 Drug-Laboratory Test Interactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Combined Hormonal Products
7.2 Effect of Combined Hormonal Products on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Effects on Vasomotor Symptoms
14.2 Effects on the Endometrium
14.3 Effects on Uterine Bleeding or Spotting
14.4 Effect on Bone Mineral Density
14.5 Women's Health Initiative Studies
14.6 Women's Health Initiative Memory Study
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
17.1 Abnormal Vaginal Bleeding
17.2 Possible Serious Adverse Reactions with Estrogen Plus Progestin Therapy
17.3 Possible Less Serious but Common Adverse Reactions with Estrogen Plus Progestin Therapy
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CARDIOVASCULAR DISORDERS, BREAST CANCER, ENDOMETRIAL CANCER AND PROBABLE DEMENTIA
Estrogen Plus Progestin Therapy
Cardiovascular Disorders and Probable Dementia
Estrogen plus progestin therapy should not be used for the prevention of cardiovascular disease or dementia [see Warnings and Precautions (5.1, 5.3), and Clinical Studies (14.5, 14.6)].
The Women's Health Initiative (WHI) estrogen plus progestin substudy reported an increased risk of deep vein thrombosis (DVT), pulmonary embolism (PE), stroke and myocardial infarction (MI) in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg] combined with medroxyprogesterone acetate (MPA) [2.5 mg], relative to placebo [see Warnings and Precautions (5.1), and Clinical Studies (14.5)].
The WHI Memory Study (WHIMS) estrogen plus progestin ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women [see Warnings and Precautions (5.3), Use in Specific Populations (8.5), and Clinical Studies (14.6)].
Breast Cancer
The WHI estrogen plus progestin substudy also demonstrated an increased risk of invasive breast cancer [see Warnings and Precautions (5.2), and Clinical Studies (14.5)].
In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and MPA and other combinations and dosage forms of estrogens and progestins.
Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Estrogen-Alone Therapy
Endometrial Cancer
There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding [see Warnings and Precautions (5.2)].
Cardiovascular Disorders and Probable Dementia
Estrogen-alone therapy should not be used for the prevention of cardiovascular disease or dementia [see Warnings and Precautions (5.1, 5.3), and Clinical Studies (14.5, 14.6)].
The WHI estrogen-alone substudy reported increased risks of stroke and DVT in postmenopausal women (50 to 79 years of age) during 7.1 years of treatment with daily oral CE (0.625 mg)-alone, relative to placebo [see Warnings and Precautions (5.1), and Clinical Studies (14.5)].
The WHIMS estrogen-alone ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 5.2 years of treatment with daily CE (0.625 mg)-alone, relative to placebo. It is unknown whether this finding applies to younger postmenopausal women [see Warnings and Precautions (5.3), Use in Specific Populations (8.5), and Clinical Studies (14.6)].
In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and other dosage forms of estrogens. Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Use of estrogen-alone, or in combination with a progestin, should be with the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Postmenopausal women should be re-evaluated periodically as clinically appropriate to determine if treatment is still necessary.
-
3 DOSAGE FORMS AND STRENGTHS
The following two strengths of norethindrone acetate and ethinyl estradiol tablets are available:
- 0.5 mg/2.5 mcg: Each round light yellow tablet contains 0.5 mg norethindrone acetate and 2.5 mcg ethinyl estradiol; debossed with N1 on one side.
- 1 mg/5 mcg: Each round white tablet contains 1 mg norethindrone acetate and 5 mcg ethinyl estradiol; debossed with N2 on one side.
-
4 CONTRAINDICATIONS
Norethindrone acetate and ethinyl estradiol tablets are contraindicated in women with any of the following conditions:
- Undiagnosed abnormal genital bleeding
- Known, suspected, or history of breast cancer
- Known or suspected estrogen-dependent neoplasia
- Active DVT, PE or a history of these conditions
- Active arterial thromboembolic disease (for example, stroke and MI), or a history of these conditions
- Known anaphylactic reaction or angioedema to Norethindrone acetate and Ethinyl estradiol tablets.
- Known liver impairment or disease
- Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders
- Known or suspected pregnancy
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Disorders
An increased risk of PE, DVT, stroke, and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
Stroke
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years) [see Clinical Studies (14.5)]. The increase in risk was demonstrated after the first year and persisted.1 Should a stroke occur or be suspected, estrogen plus progestin therapy should be discontinued immediately.
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily conjugated estrogens CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). The increase in risk was demonstrated in year 1 and persisted [see Clinical Studies (14.5)]. Should a stroke occur or be suspected, estrogen-alone therapy should be discontinued immediately.
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
Coronary Heart Disease
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of coronary heart disease (CHD) events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women-years).1 An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 [see Clinical Studies (14.5)].
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo2 [see Clinical Studies (14.5)].
Subgroup analyses of women 50 to 59 years of age suggest a statistically non-significant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women with less than 10 years since menopause (8 versus 16 per 10,000 woman-years).1
In postmenopausal women with documented heart disease (n = 2,763), average 66.7 years of age, in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study [HERS]), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand, three hundred and twenty-one (2,321) women from the original HERS trial agreed to participate in an open label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in HERS, HERS II, and overall.
Venous Thromboembolism
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women-years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted3 [see Clinical Studies (14.5)]. Should a VTE occur or be suspected, estrogen plus progestin therapy should be discontinued immediately.
In the WHI estrogen-alone substudy, the risk of VTE was increased for women receiving daily CE (0.625 mg) alone compared to placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women-years). The increase in VTE risk was demonstrated during the first 2 years4 [see Clinical Studies (14.5)]. Should a VTE occur or be suspected, estrogen-alone therapy should be discontinued immediately. If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism or during periods of prolonged immobilization.
5.2 Malignant Neoplasms
Breast Cancer
The most important randomized clinical trial providing information about breast cancer in estrogen plus progestin users is the WHI substudy of daily CE (0.625 mg) plus MPA (2.5 mg). After a mean follow-up of 5.6 years, the estrogen plus progestin substudy reported an increased risk of invasive breast cancer in women who took daily CE plus MPA. In this substudy, prior use of estrogen-alone or estrogen plus progestin therapy was reported by 26 percent of the women. The relative risk of invasive breast cancer was 1.24, and the absolute risk was 41 versus 33 cases per 10,000 women-years for CE plus MPA compared with placebo [see Clinical Studies (14.5)]. Among women who reported prior use of hormone therapy, the relative risk of invasive breast cancer was 1.86 and the absolute risk was 46 versus 25 cases per 10,000 women-years for CE plus MPA compared with placebo. Among women who reported no prior use of hormone therapy, the relative risk of invasive breast cancer was 1.09, and the absolute risk was 40 versus 36 cases per 10,000 women-years for CE plus MPA compared with placebo. In the same substudy, invasive breast cancers were larger, were more likely to be node positive, and were diagnosed at a more advanced stage in the CE (0.625 mg) plus MPA (2.5 mg) group compared with the placebo group. Metastatic disease was rare with no apparent difference between the two groups. Other prognostic factors, such as histologic subtype, grade and hormone receptor status did not differ between the groups5 [see Clinical Studies (14.5)].
The most important randomized clinical trial providing information about breast cancer in estrogen-alone users is the WHI substudy of daily CE (0.625 mg)-alone. In the WHI estrogen-alone substudy, after an average follow up of 7.1 years, daily CE-alone was not associated with an increased risk of invasive breast cancer (relative risk [RR] 0.80 [see Clinical Studies (14.5)]. Consistent with the WHI clinical trials, observational studies have also reported an increased risk of breast cancer for estrogen plus progestin therapy, and a smaller increased risk for estrogen-alone therapy, after several years of use. The risk increased with duration of use, and appeared to return to baseline over about 5 years after stopping treatment (only the observational studies have substantial data on risk after stopping). Observational studies also suggest that the risk of breast cancer was greater, and became apparent earlier, with estrogen plus progestin therapy as compared to estrogen-alone therapy. However, these studies have not found significant variation in the risk of breast cancer among different estrogen plus progestin combinations, doses, or routes of administration.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors and prior mammogram results.
Endometrial Cancer
Endometrial hyperplasia (a possible precursor of endometrial cancer) has been reported to occur at a rate of approximately 1 percent or less with norethindrone acetate and ethinyl estradiol tablets.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among unopposed estrogen users is about 2 to 12 times greater than in non-users, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use, with increased risks of 15- to 24-fold for 5 to 10 years or more. This risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding.
There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy in postmenopausal women has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
Ovarian Cancer
The WHI estrogen plus progestin substudy reported a statistically non-significant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI, 0.77-3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years] vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
5.3 Probable Dementia
In the WHIMS estrogen plus progestin ancillary study of WHI, a population of 4,532 postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo. After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21-3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years8 [see Use in Specific Populations (8.5), and Clinical Studies (14.6)].
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo.
After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83-2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years8 [see Use in Specific Populations (8.5), and Clinical Studies (14.6)].
When data from the two populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19-2.60). Since both ancillary substudies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women8 [see Use in Specific Populations (8.5), and Clinical Studies (14.6)].
5.4 Gallbladder Disease
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
5.5 Hypercalcemia
Estrogen administration may lead to severe hypercalcemia in women with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
5.6 Visual Abnormalities
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
5.7 Addition of a Progestin When a Woman Has Not Had a Hysterectomy
Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
There are, however, possible risks that may be associated with the use of progestins with estrogens compared to estrogen-alone regimens. These include an increased risk of breast cancer.
5.8 Elevated Blood Pressure
In a small number of case reports, substantial increases in blood pressure have been attributed to idiosyncratic reactions to estrogens. In a large, randomized, placebo-controlled clinical trial, a generalized effect of estrogen therapy on blood pressure was not seen.
5.9 Hypertriglyceridemia
In women with pre-existing hypertriglyceridemia, estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis. Consider discontinuation of treatment if pancreatitis occurs.
5.10 Hepatic Impairment and/or a Past History of Cholestatic Jaundice
Estrogens may be poorly metabolized in women with impaired liver function. For women with a history of cholestatic jaundice associated with past estrogen use or with pregnancy, caution should be exercised and in the case of recurrence, medication should be discontinued.
5.11 Hypothyroidism
Estrogen administration leads to increased thyroid-binding globulin (TBG) levels. Women with normal thyroid function can compensate for the increased TBG by making more thyroid hormone, thus maintaining free T4 and T3 serum concentrations in the normal range. Women dependent on thyroid hormone replacement therapy who are also receiving estrogen may require increased doses of their thyroid replacement therapy. These women should have their thyroid function monitored in order to maintain their free thyroid hormone levels in an acceptable range.
5.12 Fluid Retention
Estrogens plus progestins may cause some degree of fluid retention. Women with conditions that might be influenced by this factor, such as cardiac or renal impairment, warrant careful observation when estrogens plus progestins are prescribed.
5.13 Hypocalcemia
Estrogen therapy should be used with caution in women with hypoparathyroidism as estrogen-induced hypocalcemia may occur.
5.14 Exacerbation of Endometriosis
A few cases of malignant transformation of residual endometrial implants have been reported in women treated post-hysterectomy with estrogen-alone therapy. For women known to have residual endometriosis post- hysterectomy, the addition of progestin should be considered.
5.15 Hereditary Angioedema
Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema.
5.16 Exacerbation of Other Conditions
Estrogen therapy may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraine, porphyria, systemic lupus erythematosus and hepatic hemangiomas, and should be used with caution in women with these conditions.
5.17 Laboratory Tests
Serum follicle stimulating hormone (FSH) and estradiol levels have not been shown to be useful in the management of moderate to severe vasomotor symptoms.
5.18 Drug-Laboratory Test Interactions
Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex, and beta-thromboglobulin; decreased levels of antifactor Xa and antithrombin III, decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
Increased TBG levels leading to increased circulating total thyroid hormone levels as measured by protein-bound iodine (PBI), T4 levels (by column or by radioimmunoassay) or T3 levels by radioimmunoassay. T3 resin uptake is decreased, reflecting the elevated TBG. Free T4 and free T3 concentrations are unaltered. Women on thyroid replacement therapy may require higher doses of thyroid hormone.
Other binding proteins may be elevated in serum, for example, corticosteroid binding globulin (CBG), sex hormone-binding globulin (SHBG), leading to increased total circulating corticosteroids and sex steroids, respectively. Norethindrone acetate and ethinyl estradiol tablets 1/5 was associated with an SHBG increase of 22 percent.
Free hormone concentrations, such as testosterone and estradiol, may be decreased. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-1-antitrypsin, ceruloplasmin).
Increased plasma high-density lipoprotein (HDL) and HDL2 cholesterol subfraction concentrations, reduced low-density lipoprotein (LDL) cholesterol concentration, increased triglycerides levels. Impaired glucose tolerance.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Cardiovascular Disorders [see Boxed Warning, Warnings and Precautions (5.1)].
- Malignant Neoplasms [see Boxed Warning, Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions reported by ≥5 percent of subjects in controlled clinical studies of norethindrone acetate and ethinyl estradiol tablets are shown in Table 1.
Table 1. Associated Adverse Reactions Reported by ≥ 5 Percent of Subjects by Body System* BODY SYSTEM/
Adverse ReactionNumber (Percent) of Subjects Placebo
N = 247NDAc-EE 0.5/2.5†
N = 244NDAc-EE 1/5†
N = 258- *
- The total number of subjects for each body system may be less than the number of subjects with AEs in that body system because a subject may have had more than one AE per body system
- †
- NDAc-EE 0.5/2.5 = Norethindrone Acetate - Ethinyl Estradiol 0.5 mg/2.5 mcg
NDAc-EE 1/5 = Norethindrone Acetate - Ethinyl Estradiol 1 mg/5 mcg
BODY AS A WHOLE 23 (12.8) 30 (16.9) 30 (15.7) Edema – Generalized 10 (4.0) 12 (4.9) 11 (4.3) Headache 12 (4.9) 14 (5.7) 16 (6.2) DIGESTIVE SYSTEM 8 (4.4) 17 (9.6) 25 (13.1) Abdominal Pain 3 (1.2) 13 (5.3) 14 (6.8) UROGENITAL SYSTEM 20 (11.1) 34 (19.2) 45 (23.6) Breast Pain 9 (3.6) 22 (9.0) 20 (7.8) 6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of norethindrone acetate and ethinyl estradiol tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Genitourinary System
Changes in vaginal bleeding pattern and abnormal withdrawal bleeding or flow; breakthrough bleeding; spotting; increase in size of uterine leiomyomata, vaginitis, including vaginal candidiasis; change in amount of cervical secretion; changes in cervical ectropion; ovarian cancer; endometrial hyperplasia; endometrial cancer; uterine cancer; vaginal hemorrhage; ovarian cyst; irregular menstruation; metrorrhagia; menorrhagia; dysmenorrhea; uterine enlargement.
Breasts
Tenderness, enlargement, breast pain, nipple pain, nipple discharge, galactorrhea; fibrocystic breast changes; breast cancer; breast disorder; breast mass; breast enlargement.
Cardiovascular
Deep and superficial venous thrombosis; pulmonary embolism; thrombophlebitis; thrombosis; chest pain; myocardial infarction; cerebrovascular accident (stroke); transient ischemic attack; hemiparesis; increase in blood pressure; irregular heart rate; palpitations; dyspnea.
Gastrointestinal
Nausea, vomiting; cholestatic jaundice; pancreatitis, enlargement of hepatic hemangiomas; bloating, abdominal cramps; abdominal pain; increased incidence of gallbladder disease; cholecystitis; cholelithiasis.
Skin
Chloasma or melasma that may persist when drug is discontinued; generalized erythema; erythema multiforme; erythema nodosum; hemorrhagic eruption; loss of scalp hair; hirsutism; rash, pruritus.
Central Nervous System (CNS)
Headache; migraine; dizziness; depression; chorea; nervousness; mood disturbances; irritability; exacerbation of epilepsy, dementia; paresthesia; insomnia.
Miscellaneous
Increase or decrease in weight; reduced carbohydrate tolerance; aggravation of porphyria; edema; arthralgias; leg cramps; back pain; changes in libido; urticaria, angioedema, anaphylactoid/anaphylactic reactions; hypocalcemia; exacerbation of asthma; increased triglycerides; blood glucose abnormal; fatigue; myalgia; hypersensitivity.
-
7 DRUG INTERACTIONS
No drug-drug interaction studies have been conducted for norethindrone acetate and ethinyl estradiol tablets.
7.1 Effect of Other Drugs on Combined Hormonal Products
Substances decreasing or increasing the plasma concentration of estrogen: In vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen drug metabolism. Inducers of CYP3A4 such as St. John's wort (Hypericum perforatum) preparations, phenobarbital, carbamazepine and rifampin may decrease the plasma concentration of estrogens, possibly resulting in a decrease in therapeutic effects and/or changes in the uterine bleeding profile. Inhibitors of CYP3A4 such as erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir and grapefruit juice may increase the plasma concentration of estrogens and may result in side effects. Co-administration of atorvastatin and certain hormonal products containing ethinyl estradiol increase AUC values for ethinyl estradiol approximately 20 percent. Ascorbic acid and acetaminophen may increase the plasma ethinyl estradiol concentration, possibly by inhibition of conjugation.
7.2 Effect of Combined Hormonal Products on Other Drugs
Combination hormonal products containing some synthetic estrogens (for example, ethinyl estradiol) may inhibit the metabolism of other compounds. Combination hormonal products have been shown to significantly decrease the plasma concentration of lamotrigine likely due to induction of lamotrigine glucuronidation. This may reduce seizure control; therefore, dosage adjustments of lamotrigine may be necessary.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Norethindrone acetate and ethinyl estradiol tablets should not be used during pregnancy [see Contraindications (4)]. There appears to be little or no increased risk of birth defects in children born to women who have used estrogens and progestins as an oral contraceptive inadvertently during early pregnancy.
8.3 Nursing Mothers
Norethindrone acetate and ethinyl estradiol tablets should not be used during lactation. Estrogen administration to nursing women has been shown to decrease the quantity and quality of the breast milk. Detectable amounts of estrogen and progestin have been identified in the breast milk of women receiving estrogen plus progestin therapy. Caution should be exercised when norethindrone acetate and ethinyl estradiol tablets are administered to a nursing woman.
8.4 Pediatric Use
Norethindrone acetate and ethinyl estradiol tablets are not indicated in children. Clinical studies have not been conducted in the pediatric population.
8.5 Geriatric Use
There have not been sufficient numbers of geriatric women involved in clinical studies utilizing norethindrone acetate and ethinyl estradiol tablets to determine whether those over 65 years of age differ from younger subjects in their response to norethindrone acetate and ethinyl estradiol tablets.
The Women's Health Initiative Studies
In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age [see Clinical Studies (14.5)].
In the WHI estrogen-alone substudy (daily CE [0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age [see Clinical Studies (14.5)].
The Women's Health Initiative Memory Study
In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen plus progestin or estrogen-alone when compared to placebo [see Warnings and Precautions (5.3), and Clinical Studies (14.6)].
Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women8 [see Warnings and Precautions (5.3), and Clinical Studies (14.6)].
-
10 OVERDOSAGE
Overdosage of estrogen plus progestin may cause nausea, vomiting, breast tenderness, abdominal pain, drowsiness and fatigue, and withdrawal bleeding may occur in women. Treatment of overdose consists of discontinuation of norethindrone acetate and ethinyl estradiol tablets with institution of appropriate symptomatic care.
-
11 DESCRIPTION
Norethindrone acetate and ethinyl estradiol tablets are a continuous dosage regimen of a progestin-estrogen combination for oral administration.
The following two strengths of norethindrone acetate and ethinyl estradiol tablets are available:
- 0.5 mg/2.5 mcg: Each round light yellow tablet contains 0.5 mg norethindrone acetate and 2.5 mcg ethinyl estradiol; debossed with N1 on one side.
- 1 mg/5 mcg: Each round white tablet contains 1 mg norethindrone acetate and 5 mcg ethinyl estradiol; debossed with N2 on one side.
Each white tablet also contains lactose monohydrate, pregelatinized starch, polyethylene glycol, magnesium stearate, ethyl cellulose, vitamin E, titanium dioxide, hypromelloses, macrogol/PEG, triacetin, polydextrose.
Each light yellow tablet also contains lactose monohydrate, pregelatinized starch, polyethylene glycol, magnesium stearate, ethyl cellulose, vitamin E, iron oxide yellow, iron oxide black, talc, polyvinyl alcohol, titanium dioxide, lecithin (soya).
The structural formulas are as follows.
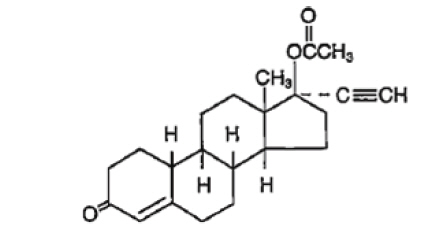
Norethindrone Acetate
[19-Norpregn-4-en-20-yn-3-one, 17-(acetyloxy)-, (17α)-];
Molecular Weight: 340.47 Molecular Formula: C22H28O3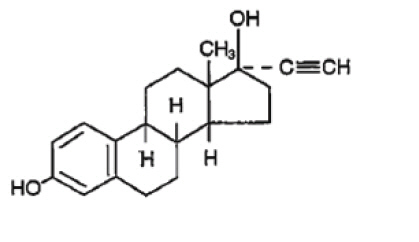
Ethinyl Estradiol
[19-Norpregna-1,3,5(10)-trien-20-yne-3,17-diol, (17α)-];
Molecular Weight: 296.41 Molecular Formula: C20H24O2 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol, at the receptor level.
The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, which is secreted by the adrenal cortex, to estrone in the peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women. The pharmacologic effects of ethinyl estradiol are similar to those of endogenous estrogens.Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, two estrogen receptors have been identified. These vary in proportion from tissue to tissue.Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and FSH through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.Progestin compounds enhance cellular differentiation and generally oppose the actions of estrogens by decreasing estrogen receptor levels, increasing local metabolism of estrogens to less active metabolites, or inducing gene products that blunt cellular responses to estrogen. Progestins exert their effects in target cells by binding to specific progesterone receptors that interact with progesterone response elements in target genes. Progesterone receptors have been identified in the female reproductive tract, breast, pituitary, hypothalamus, bone, skeletal tissue and central nervous system. Progestins produce similar endometrial changes to those of the naturally occurring hormone progesterone.
12.2 Pharmacodynamics
Currently, there are no pharmacodynamic data known for norethindrone acetate and ethinyl estradiol tablets.
12.3 Pharmacokinetics
Absorption
Norethindrone acetate (NA) is completely deacetylated to norethindrone after oral administration, and the disposition of norethindrone acetate is indistinguishable from that of orally administered norethindrone. Norethindrone acetate and ethinyl estradiol (EE) are absorbed from norethindrone acetate and ethinyl estradiol tablets, with maximum plasma concentrations of norethindrone and ethinyl estradiol generally occurring 1 to 2 hours postdose. Both are subject to first-pass metabolism after oral dosing, resulting in an absolute bioavailability of approximately 64 percent for norethindrone and 55 percent for ethinyl estradiol. Bioavailability of norethindrone acetate and ethinyl estradiol tablets is similar to that from solution for norethindrone and slightly less for ethinyl estradiol. Administration of norethindrone acetate and ethinyl estradiol tablets with a high fat meal decreases rate but not extent of ethinyl estradiol absorption. The extent of norethindrone absorption is increased by 27 percent following administration of norethindrone acetate and ethinyl estradiol tablets with food.
The full pharmacokinetic profile of norethindrone acetate and ethinyl estradiol tablets was not characterized due to assay sensitivity limitations. However, the multiple-dose pharmacokinetics were studied at a dose of 1 mg NA/10 mcg EE in 18 postmenopausal women. Mean plasma concentrations are shown below (Figure 1) and pharmacokinetic parameters are found in Table 2. Based on a population pharmacokinetic analysis, mean steady-state concentrations of norethindrone for 1 mg NA/5 mcg EE and 1/10 are slightly more than proportional to dose when compared to 0.5 mg NA/2.5 mcg EE tablets. It can be explained by higher SHBG concentrations. Mean steady-state plasma concentrations of ethinyl estradiol for the norethindrone acetate and ethinyl estradiol 0.5/2.5 tablets and norethindrone acetate and ethinyl estradiol 1/5 tablets are proportional to dose, but there is a less than proportional increase in steady-state concentrations for the NA/EE 1/10 tablet.
Figure 1. Mean Steady-State (Day 87) Plasma Norethindrone and Ethinyl Estradiol Concentrations Following Continuous Oral Administration of 1 mg NA/10 mcg EE Tablets
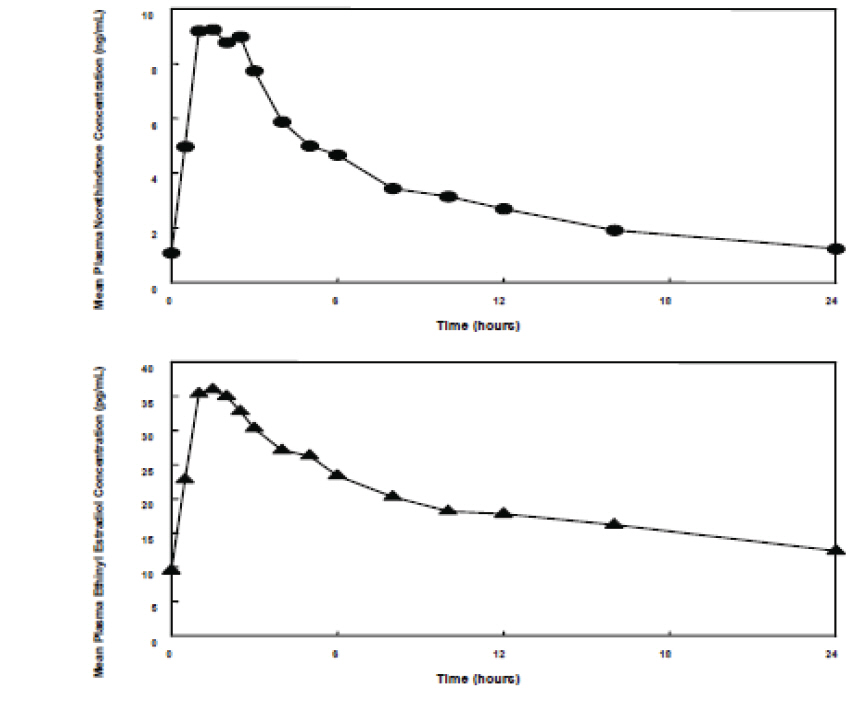
Table 2: Mean (SD) Single-Dose (Day 1) and Steady-State (Day 87) Pharmacokinetic Parameters* Following Administration of 1 mg NA/10 mcg EE Tablets Cmax tmax AUC(0-24) CL/F t½ Norethindrone ng/mL hr ng.hr/mL mL/min hr Day 1 6.0 (3.3) 1.8 (0.8) 29.7 (16.5) 588 (416) 10.3 (3.7) Day 87 10.7 (3.6) 1.8 (0.8) 81.8 (36.7) 226 (139) 13.3 (4.5) Ethinyl Estradiol pg/mL hr pg.hr/mL mL/min hr Day 1 33.5 (13.7) 2.2 (1.0) 339 (113) ND † ND † Day 87 38.3 (11.9) 1.8 (0.7) 471 (132) 383 (119) 23.9 (7.1) Based on a population pharmacokinetic analysis, average steady-state concentrations (Css) of norethindrone acetate and ethinyl estradiol for the norethindrone acetate and ethinyl estradiol 1/5 tablets are estimated to be 2.6 ng/mL and 11.4 pg/mL, respectively. Css values of norethindrone and ethinyl estradiol for the norethindrone acetate and ethinyl estradiol 0.5/2.5 tablets are estimated to be 1.1 ng/mL and 5.4 ng/mL, respectively. The pharmacokinetics of ethinyl estradiol and norethindrone acetate were not affected by age, (age range 40-62 years), in the postmenopausal population studied.
Distribution
The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Estrogens circulate in the blood largely bound to SHBG and albumin.
Volume of distribution of norethindrone and ethinyl estradiol ranges from 2 to 4 L/kg. Plasma protein binding of both steroids is extensive (greater than 95 percent); norethindrone binds to both albumin and SHBG, whereas ethinyl estradiol binds only to albumin. Although ethinyl estradiol does not bind to SHBG, it induces SHBG synthesis.
Metabolism
Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is a major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the intestine followed by reabsorption. In postmenopausal women, a significant proportion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens.
Norethindrone undergoes extensive biotransformation, primarily via reduction, followed by sulfate and glucuronide conjugation. The majority of metabolites in the circulation are sulfates, with glucuronides accounting for most of the urinary metabolites. A small amount of norethindrone acetate is metabolically converted to ethinyl estradiol, such that exposure to ethinyl estradiol following administration of 1 mg of norethindrone acetate is equivalent to oral administration of 2.8 mcg ethinyl estradiol. Ethinyl estradiol is also extensively metabolized, both by oxidation and by conjugation with sulfate and glucuronide. Sulfates are the major circulating conjugates of ethinyl estradiol and glucuronides predominate in urine. The primary oxidative metabolite is 2-hydroxy ethinyl estradiol, formed by the CYP3A4 isoform of cytochrome P450. Part of the first pass metabolism of ethinyl estradiol is believed to occur in gastrointestinal mucosa. Ethinyl estradiol may undergo enterohepatic circulation.
Excretion
Estradiol, estrone, and estriol are excreted in the urine along with glucuronide and sulfate conjugates.
Norethindrone and ethinyl estradiol are excreted in both urine and feces, primarily as metabolites. Plasma clearance values for norethindrone and ethinyl estradiol are similar (approximately 0.4 L/hr/kg). Steady-state elimination half-lives of norethindrone and ethinyl estradiol following administration of 1 mg NA/10 mcg EE tablets are approximately 13 hours and 24 hours, respectively.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Effects on Vasomotor Symptoms
A 12-week placebo-controlled, multicenter, randomized clinical trial was conducted in 266 symptomatic women who had at least 56 moderate to severe hot flushes during the week prior to randomization. On average, patients had 12 hot flushes per day upon study entry.
A total of 66 women were randomized to receive norethindrone acetate and ethinyl estradiol 1/5 and 66 women were randomized to the placebo group. Norethindrone acetate and ethinyl estradiol 1/5 was shown to be statistically better than placebo at weeks 4, and 12 for relief of the frequency of moderate to severe vasomotor symptoms (see Table 3). In Table 4, norethindrone acetate and ethinyl estradiol 1/5 was shown to be statistically better than placebo at weeks 4 and 12 for relief of the severity of moderate to severe vasomotor symptoms.
Table 3: Mean Change from Baseline in the Number of Moderate to Severe Vasomotor Symptoms per Week-ITT Population, LOCF Visit Placebo
(N=66)Norethindrone Acetate and Ethinyl Estradiol 0.5/2.5
(N=67)Norethindrone Acetate and Ethinyl Estradiol 1/5
(N=66)ITT = intent to treat; LOCF = last observation carried forward; CI = confidence interval 2 randomized subjects (1 in Placebo and 1 in norethindrone acetate and ethinyl estradiol) did not return diaries. - *
- The baseline number of moderate to severe vasomotor symptoms (MSVS) is the weekly average number of MSVS during the two week prerandomization observation period.
- †
- Denotes statistical significance at the 0.05 level
- ‡
- ANCOVA - Analysis of Covariance model where the observation variable is change from baseline; independent variables include treatment, center and baseline as covariate. The 95% CI -Mann-Whitney confidence interval for the difference between means (not stratified by center).
Baseline * Mean (SD) 76.5 (21.4) 77.6 (26.5) 70.0 (16.6) Week 4 Mean (SD) 39.4 (27.6) 30.2 (26.1) 20.4 (22.7) Mean Change from Baseline (SD) -37.0 (26.6) -47.4† (26.1) -49.6† (22.1) p-Value vs. Placebo (95% CI) ‡ 0.041 ( -20.0, -1.0) <0.001 ( -22.0, -6.0) Week 12 Mean (SD) 31.1 (27.0) 13.8 (20.4) 11.3 (18.9) Mean Change from Baseline (SD) -45.3 (30.2) -63.8† (27.5) -58.7† (23.1) p-Value vs. Placebo (95% CI) ‡ <0.001 ( -27.0, -7.0) <0.001 ( -25.0, -5.0) Table 4: Mean Change from Baseline in the Daily Severity Score of Moderate to Severe Vasomotor Symptoms per Week - ITT Population, LOCF Visit Placebo
(N=66)Norethindrone Acetate and Ethinyl Estradiol 0.5/2.5
(N=67)Norethindrone Acetate and Ethinyl Estradiol 1/5
(N=66)ITT = intent to treat; LOCF = last observation carried forward; CI = confidence interval 2 randomized subjects (1 in Placebo and 1 in norethindrone acetate and ethinyl estradiol) did not return diaries. - *
- The baseline severity of moderate to severe vasomotor symptoms (MSVS) is the daily severity score of MSVS during the two week pre-randomization observation period.
- †
- Denotes statistical significance at the 0.05 level
- ‡
- ANCOVA - Analysis of Covariance model where the observation variable is change from baseline; independent variables include treatment, center and baseline as covariate. The 95% CI - Mann-Whitney confidence interval for the difference between means (not stratified by center).
Baseline * Mean (SD) 2.49 (0.26) 2.48 (0.22) 2.47 (0.23) Week 4 Mean (SD) 2.13 (0.74) 1.88 (0.89) 1.45 (1.03) Mean Change from Baseline (SD) -0.36 (0.68) -0.59 (0.83) -1.02† (1.06) p-Value vs. Placebo (95% CI) ‡ - 0.130 ( -0.3, 0.0) <0.001 ( -0.9, -0.2) Week 5 Mean (SD) 2.06 (0.79) 1.68 (0.99) 1.23 (1.03) Mean Change from Baseline (SD) -0.44 (0.74) -0.80† (0.94) -1.24† (1.07) p-Value vs. Placebo (95% CI) ‡ - 0.041 ( -0.4, -0.0) <0.001 ( -1.2, -0.3) Week 12 Mean (SD) 1.82 (1.03) 1.22 (1.11) 1.02 (1.16) Mean Change from Baseline (SD) -0.67 (1.02) -1.26† (1.08) -1.45† (1.19) p-Value vs. Placebo (95% CI) ‡ - 0.002 ( -0.9, -0.2) <0.001 ( -1.4, -0.3) 14.2 Effects on the Endometrium
A 2-year, placebo-controlled, multicenter, randomized clinical trial was conducted to determine the safety and efficacy of norethindrone acetate and ethinyl estradiol on maintaining bone mineral density, protecting the endometrium, and to determine effects on lipids. A total of 1265 women were enrolled and randomized to either placebo, 0.2 mg NA/1 mcg EE, norethindrone acetate and ethinyl estradiol 0.5/2.5, norethindrone acetate and ethinyl estradiol 1/5 and 1 mg NA/10 mcg EE or matching unopposed EE doses (1, 2.5, 5, or 10 mcg) for a total of 9 treatment groups. All participants received 1000 mg of calcium supplementation daily. Of the 1265 women randomized to the various treatment arms of this study, 137 were randomized to placebo, 146 to norethindrone acetate and ethinyl estradiol 1/5, 136 to norethindrone acetate and ethinyl estradiol 0.5/2.5 and 141 to EE 5 mcg and 137 to EE 2.5 mcg. Of these, 134 placebo, 143 norethindrone acetate and ethinyl estradiol 1/5, 136 norethindrone acetate and ethinyl estradiol 0.5/2.5,139 EE 5 mcg and 137 EE 2.5 mcg had a baseline endometrial result. Baseline biopsies were classified as normal (in approximately 95% of subjects), or insufficient tissue (in approximately 5% of subjects). Follow-up biopsies were obtained in approximately 70-80% of patients in each arm after 12 and 24 months of therapy. Results for norethindrone acetate and ethinyl estradiol 1/5 and appropriate comparators are shown in Table 5.
Table 5: Endometrial Biopsy Results After 12 and 24 Months of Treatment (CHART Study, 376 -359) Endometrial Status Placebo Norethindrone Acetate and Ethinyl Estradiol EE Alone 0.5/2.5 1/5 2.5 mcg 5 mcg Number of Patients Biopsied at Baseline N= 134 N=136 N= 143 N=137 N=139 - *
- All patients with endometrial hyperplasia were carried forward for all time points
MONTH 12 (% Patients) Patients Biopsied (%) 113 (84) 103 (74) 110 (77) 100 (73) 114 (82) Insufficient Tissue 30 34 45 20 20 Atrophic Tissue 60 41 41 15 2 Proliferative Tissue 23 28 24 65 91 Endometrial Hyperplasia* 0 0 0 0 1 MONTH 24 (% Patients) Patients Biopsied (%) 94 (70) 99 (73) 102 (71) 89 (65) 107 (77) Insufficient Tissue 35 42 37 23 17 Atrophic Tissue 38 30 33 6 2 Proliferative Tissue 20 27 32 60 86 Endometrial Hyperplasia* 1 0 0 0 2 14.3 Effects on Uterine Bleeding or Spotting
The cumulative incidence of amenorrhea, defined as no bleeding or spotting obtained from subject recall, was evaluated over 12 months for norethindrone acetate and ethinyl estradiol (NA/EE) 1/5 and placebo arms. Results are shown in Figure 2.
Figure 2. Patients With Cumulative Amenorrhea Over Time: Intent-to-Treat Population, Last Observation Carried Forward 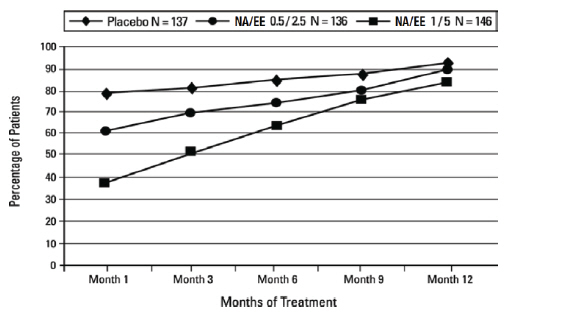
14.4 Effect on Bone Mineral Density
In the 2 year study, trabecular bone mineral density (BMD) was assessed at lumbar spine using quantitative computed tomography. A total of 419 postmenopausal primarily Caucasian women, aged 40 to 64 years, with intact uteri and non-osteoporotic bone mineral densities were randomized (1:1:1) to norethindrone acetate and ethinyl estradiol 1/5, norethindrone acetate and ethinyl estradiol 0.5/2.5 or placebo. Approximately 75 percent of the subjects in each group completed the two-year study. All patients received 1000 mg calcium in divided doses. Vitamin D was not supplemented.
As shown in Figure 3, women treated with norethindrone acetate and ethinyl estradiol 1/5 had an average increase of 3.1percent in lumbar spine BMD from baseline to Month 24. Women treated with placebo had average decreases of –6.3 percent in spinal BMD from baseline to Month 24. The differences in the changes from baseline to Month 24 in the norethindrone acetate and ethinyl estradiol 1/5 group compared with the placebo group were statistically significant.
Figure 3: Mean Percent Change (+ SE) From Baseline in Volumetric Bone Mineral Density* at Lumbar Spine Measured by Quantitative Computed Tomography after 12 and 24 Months of Treatment (Intent-to-Treat Population)
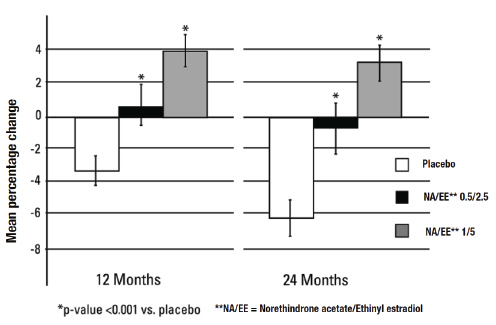
*It should be noted that when measured by QCT, BMD gains and losses are greater than when measured by dual X-ray absorptiometry (DXA). Therefore, the differences in the changes in BMD between the placebo and active drug treated groups will be larger when measured by QCT compared with DXA. Changes in BMD measured by DXA should not be compared with changes in BMD measured by QCT.
14.5 Women's Health Initiative Studies
The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of CHD (defined as nonfatal MI, silent MI and CHD death), with invasive breast cancer as the primary adverse outcome. A "global index" included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other cause. The study did not evaluate the effects of CE plus MPA or CE-alone on menopausal symptoms.
WHI Estrogen Plus Progestin Substudy
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of invasive breast cancer and cardiovascular events exceeded the specified benefits included in the "global index". The absolute excess risk of events included in the "global index" was 19 per 10,000 women-years. For those outcomes included in the WHI "global index" that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women-years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reductions per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the CE plus MPA substudy, which included 16,608 women (average 63 years of age, range 50 to 79; 83.9 percent White, 6.8 percent Black, 5.4 percent Hispanic, 3.9 percent Other), are presented in Table 6. These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
Table 6. Relative and Absolute Risk Seen in the Estrogen Plus Progestin Substudy of WHI at an Average of 5.6 years*,† Event Relative Risk
CE/MPA vs placebo
(95 pecent nCI ‡)CE/MPA
n = 8506Placebo
n = 8102Absolute Risk per 10,000 Women -years - *
- Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi
- †
- Results are based on centrally adjudicated data.
- ‡
- Nominal confidence intervals unadjusted for multiple looks and multiple comparisons.
- §
- Not included in "global index".
- ¶
- Includes metastatic and non -metastatic breast cancer with the exception of in situ cancer.
- #
- All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.
- Þ
- A subset of the events was combined in a "global index" defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other uses.
CHD events 1.23 (0.99 -1.53) 41 34 Non-fatal MI 1.28 (1.00 -1.63) 31 25 CHD death 1.10 (0.70 -1.75) 8 8 All strokes 1.31 (1.03 -1.68) 33 25 Ischemic stroke 1.44 (1.09 -1.90) 26 18 Deep vein thrombosis § 1.95 (1.43 -2.67) 26 13 Pulmonary embolism 2.13 (1.45 -3.11) 18 8 Invasive breast cancer ¶ 1.24 (1.01 -1.54) 41 33 Colorectal cancer 0.61 (0.42 -0.87) 10 16 Endometrial cancer § 0.81 (0.48 -1.36) 6 7 Cervical cancer § 1.44 (0.47 -4.42) 2 1 Hip fracture 0.67 (0.47 -0.96) 11 16 Vertebral fractures § 0.65 (0.46 -0.92) 11 17 Lower arm/wrist fractures § 0.71 (0.59 -0.85) 44 62 Total fractures § 0.76 (0.69 -0.83) 152 199 Overall Mortality ‡, # 1.00 (0.83 -1.19) 52 52 Global Index Þ 1.13 (1.02 -1.25) 184 165 Timing of the initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age, a non-significant trend toward reduced risk for overall mortality [hazard ratio (HR) 0.69 (95 percent CI, 0.44-1.07)].
WHI Estrogen-Alone Substudy
The WHI estrogen-alone substudy was also stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risks and benefits of estrogen-alone in predetermined primary endpoints.
Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50 to 79; 75.3 percent White, 15.1 percent Black, 6.1 percent Hispanic, 3.6 percent Other), after an average follow-up of 7.1 years, are presented in Table 7.
Table 7. Relative and Absolute Risk Seen in the Estrogen-Alone Substudy of WHI* Event Relative Risk
CE vs placebo
(95 percent nCI † )CE
n = 5,310Placebo
n = 5,429Absolute Risk per 10,000 Women -years - *
- Adapted from numerous WHI publications. WHI publications can be viewed at www.nblbi.nih.gov/whi.
- †
- Nominal confidence intervals unadjusted for multiple looks and multiple comparisons.
- ‡
- Results are based on centrally adjudicated data for an average follow -up of 7.1 years.
- §
- Not included in "global index".
- ¶
- Results are based on an average follow -up of 6.8 years.
- #
- All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.
- Þ
- A subset of the events was combined in a "global index" defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes.
CHD events ‡ 0.95 (0.78 -1.16) 54 57 Non -fatal MI ‡ 0.91 (0.73 -1.14) 40 43 CHD death ‡ 1.01 (0.71 -1.43) 16 16 All strokes ‡ 1.33 (1.05 -1.68) 45 33 Ischemic stroke ‡ 1.55 (1.19 -2.01) 38 25 Deep vein thrombosis ‡,§ 1.47 (1.06 -2.06) 23 15 Pulmonary embolism ‡ 1.37 (0.90 -2.07) 14 10 Invasive breast cancer ‡ 0.80 (0.62 -1.04) 28 34 Colorectal cancer ¶ 1.08 (0.75 -1.55) 17 16 Hip fracture ‡ 0.65 (0.45 -0.94) 12 19 Vertebral fractures ‡,§ 0.64 (0.44 -0.93) 11 18 Lower arm/wrist fractures ‡,§ 0.58 (0.47 -0.72) 35 59 Total fractures ‡,§ 0.71 (0.64 -0.80) 144 197 Deaths due to other causes¶,# 1.08 (0.88 -1.32) 53 50 Overall Mortality ‡,§ 1.04 (0.88 -1.22) 79 75 Global Index Þ 1.02 (0.92 -1.13) 206 201 For those outcomes included in the WHI "global index" that reached statistical significance, the absolute excess risk per 10,000 women-years in the group treated with CE-alone were 12 more strokes, while the absolute risk reduction per 10,000 women-years was 7 fewer hip fractures.9 The absolute excess risk of events included in the "global index" was a non-significant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality. No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years (see Table 7). Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant difference in distribution of stroke subtype or severity, including fatal strokes, in women receiving CE-alone compared to placebo. Estrogen-alone therapy increased the risk of ischemic stroke, and this excess was present in all subgroups of women examined10 (see Table 7).
Timing of the initiation of estrogen-alone therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen-alone substudy stratified by age, showed in women 50 to 59 years of age a non-significant trend toward reduced risk for CHD [HR 0.63 (95 percent CI, 0.36-1.09)] and overall mortality [HR 0.71 (95 percent CI, 0.46-1.11)].
14.6 Women's Health Initiative Memory Study
The WHIMS estrogen plus progestin ancillary study of WHI enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age, 35 percent were 70 to 74 years of age, and 18 percent were 75 years of age and older) to evaluate the effects of CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21-3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 per 10,000 women-years. Probable dementia as defined in this study included Alzheimer's disease (AD), vascular dementia (VaD) and mixed types (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger post-menopausal women [see Warnings and Precautions (5.3), and Use in Specific Populations (8.5)].
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age (45 percent were 65 to 69 years of age; 36 percent were 70 to 74 years of age; 19 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83-2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in this study included AD, VaD and mixed types (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women [see Warnings and Precautions (5.3), and Use in Specific Populations (8.5)].
When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19-2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women [see Warnings and Precautions (5.3), and Use in Specific Populations (8.5)].
-
15 REFERENCES
- Rossouw JE, et al. Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause. JAMA. 2007;297:1465-1477.
- Hsia J, et al. Conjugated Equine Estrogens and Coronary Heart Disease. Arch Int Med. 2006;166:357-365.
- Cushman M, et al. Estrogen Plus Progestin and Risk of Venous Thrombosis. JAMA. 2004;292:1573-1580.
- Curb JD, et al. Venous Thrombosis and Conjugated Equine Estrogen in Women Without a Uterus. Arch Int Med. 2006;166:772-780.
- Chlebowski RT, et al. Influence of Estrogen Plus Progestin on Breast Cancer and Mammography in Healthy Postmenopausal Women. JAMA. 2003;289:3234-3253.
- Stefanick ML, et al. Effects of Conjugated Equine Estrogens on Breast Cancer and Mammography Screening in Postmenopausal Women With Hysterectomy. JAMA. 2006;295:1647-1657.
- Anderson GL, et al. Effects of Estrogen Plus Progestin on Gynecologic Cancers and Associated Diagnostic Procedures. JAMA. 2003;290:1739-1748.
- Shumaker SA, et al. Conjugated Equine Estrogens and Incidence of Probable Dementia and Mild Cognitive Impairment in Postmenopausal Women. JAMA. 2004;291:2947-2958.
- Jackson RD, et al. Effects of Conjugated Equine Estrogen on Risk of Fractures and BMD in Postmenopausal Women With Hysterectomy: Results From the Women's Health Initiative Randomized Trial. J Bone Miner Res. 2006;21:817-828.
- Hendrix SL, et al. Effects of Conjugated Equine Estrogen on Stroke in the Women's Health Initiative. Circulation. 2006;113:2425-2434.
-
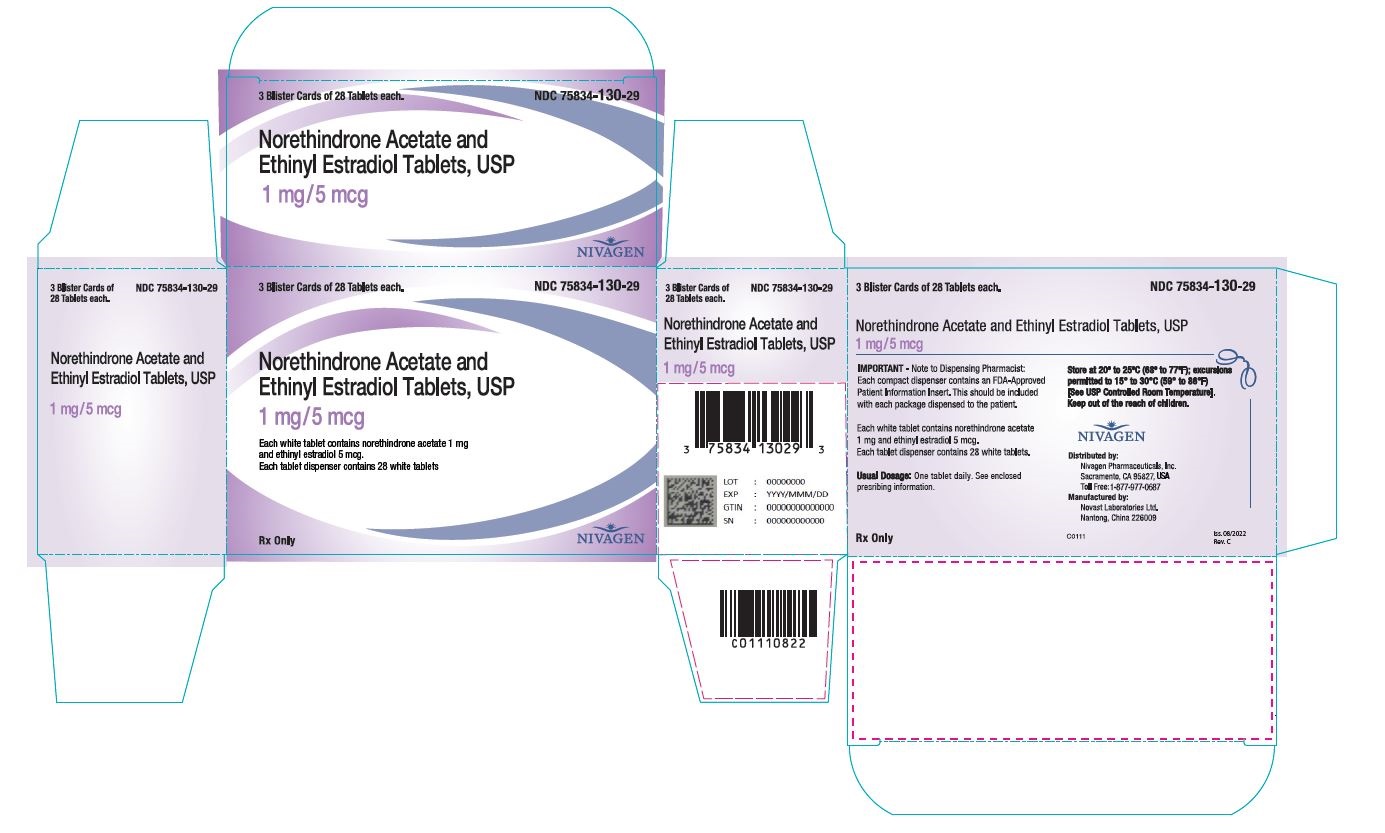
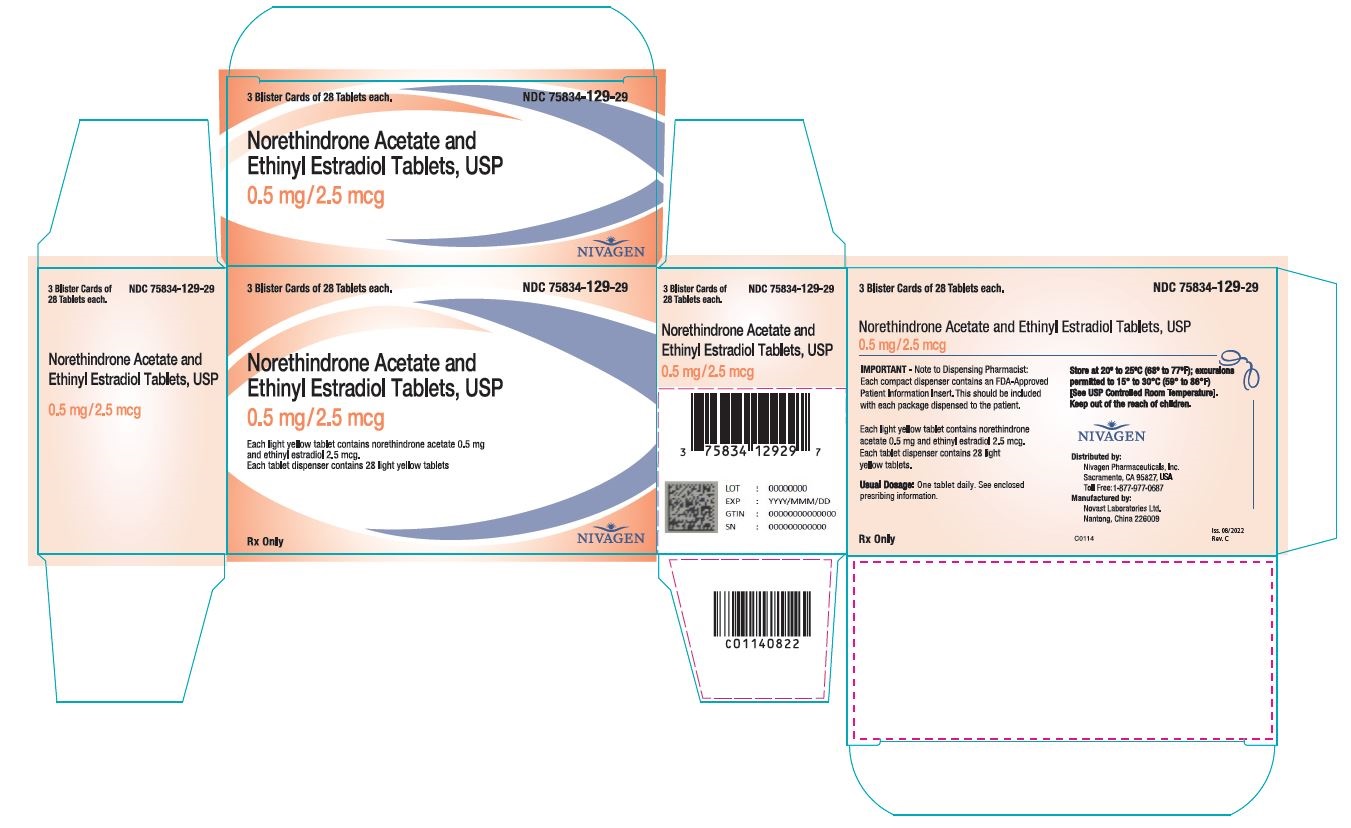
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Norethindrone acetate and ethinyl estradiol tablets are available in the following strength and package sizes:
1. 75834-129-84 Blister card of 28 round light yellow tablets with 0.5 mg norethindrone acetate and 2.5 mcg ethinyl estradiol 2. 75834-129-29 Carton containing 3 × 28 tablet blister cards each in a plastic compact. Each blister card contains 28 round light yellow tablets with 0.5 mg norethindrone acetate and 2.5 mcg ethinyl estradiol 3. 75834-129-90 Bottle of 90 round light yellow tablets with 0.5 mg norethindrone acetate and 2.5 mcg ethinyl estradiol 4. 75834-130-84 Blister card of 28 round white tablets with 1 mg norethindrone acetate and 5 mcg ethinyl estradiol 5. 75834-130-29 Carton containing 3 × 28 tablet blister cards each in a plastic compact. Each blister card contains 28 round white tablets with 1 mg norethindrone acetate and 5 mcg ethinyl estradiol 6. 75834-130-90 Bottle of 90 round white tablets with 1 mg norethindrone acetate and 5 mcg ethinyl estradiol. -
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information)
17.1 Abnormal Vaginal Bleeding
Inform postmenopausal women of the importance of reporting abnormal vaginal bleeding to their healthcare provider as soon as possible [see Warnings and Precautions (5.2)].
17.2 Possible Serious Adverse Reactions with Estrogen Plus Progestin Therapy
Inform postmenopausal women of possible serious adverse reactions of estrogen plus progestin therapy including Cardiovascular Disorders, Malignant Neoplasms, and Probable Dementia [see Warnings and Precautions (5.1, 5.2, 5.3)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
Patient Information
Norethindrone Acetate and Ethinyl Estradiol Tablets USP
0.5 mg/2.5 mcg and 1 mg/5 mcgRead this Patient Information before you start taking norethindrone acetate and ethinyl estradiol tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your menopausal symptoms or your treatment.
What is the most important information I should know about norethindrone acetate and ethinyl estradiol tablets (a combination of estrogen and progestin)?
- Do not use estrogens with progestins to prevent heart disease, heart attacks, strokes or dementia (decline of brain function).
- Using estrogens with progestins may increase your chances of getting a heart attack, strokes, breast cancer, or blood clots.
- Using estrogens with progestins may increase your chance of getting dementia, based on a study of women 65 years of age or older.
- Do not use estrogen-alone to prevent heart disease, heart attacks, strokes or dementia.
- Using estrogen-alone may increase your chance of getting cancer of the uterus (womb).
- Using estrogen-alone may increase your chances of getting strokes or blood clots.
- Using estrogen-alone may increase your chance of getting dementia, based on a study of women 65 years of age or older.
- You and your healthcare provider should talk regularly about whether you still need treatment with norethindrone acetate and ethinyl estradiol tablets.
What are norethindrone acetate and ethinyl estradiol tablets?
Norethindrone acetate and ethinyl estradiol tablets are a prescription medicine that contains two kinds of hormones, an estrogen and a progestin.
What are norethindrone acetate and ethinyl estradiol tablets used for?
Norethindrone acetate and ethinyl estradiol tablets are used after menopause to:
-
Reduce moderate to severe hot flushes
Estrogens are hormones made by a woman's ovaries. The ovaries normally stop making estrogens when a woman is between 45 and 55 years old. This drop in body estrogen levels causes the "change of life" or menopause, the end of monthly menstrual periods. Sometimes both ovaries are removed during an operation before natural menopause takes place. The sudden drop in estrogen levels causes "surgical menopause".
When estrogen levels begin dropping, some women get very uncomfortable symptoms, such as feelings of warmth in the face, neck, and chest, or sudden intense episodes of heat and sweating ("hot flashes" or "hot flushes"). In some women the symptoms are mild, and they will not need to take estrogens. In other women, symptoms can be more severe. -
Help reduce your chances of getting osteoporosis (thin weak bones)
If you use norethindrone acetate and ethinyl estradiol tablets only to prevent osteoporosis from menopause, talk with your healthcare provider about whether a different treatment or medicine without estrogens might be better for you. You and your healthcare provider should talk regularly about whether you still need treatment with norethindrone acetate and ethinyl estradiol tablets.
Who should not take norethindrone acetate and ethinyl estradiol tablets?
Do not take norethindrone acetate and ethinyl estradiol tablets if you have had your uterus (womb) removed (hysterectomy).
Norethindrone acetate and ethinyl estradiol tablets contain a progestin to decrease the chance of getting cancer of the uterus. If you do not have a uterus, you do not need a progestin and you should not take norethindrone acetate and ethinyl estradiol tablets.
Do not take norethindrone acetate and ethinyl estradiol tablets if you:
-
have unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb).
Your healthcare provider should check any unusual vaginal bleeding to find out the cause. -
currently have or have had certain cancers.
Estrogens may increase the chance of getting certain types of cancers, including cancer of the breast or uterus. If you have or have had cancer, talk with your healthcare provider about whether you should take norethindrone acetate and ethinyl estradiol tablets - had a stroke or heart attack
- currently have or have had blood clots
- currently have or have had liver problems
- have been diagnosed with a bleeding disorder
-
are allergic to norethindrone acetate and ethinyl estradiol tablets or any of its ingredients.
See the list of ingredients in norethindrone acetate and ethinyl estradiol tablets at the end of this leaflet. -
think you may be pregnant
Norethindrone acetate and ethinyl estradiol tablets are not for pregnant women. If you think you may be pregnant, you should have a pregnancy test and know the results. Do not take norethindrone acetate and ethinyl estradiol tablets if the test is positive and talk to your healthcare provider.
What should I tell my healthcare provider before I take norethindrone acetate and ethinyl estradiol tablets?
Before you take norethindrone acetate and ethinyl estradiol tablets, tell your healthcare provider if you:
-
have any unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb).
Your healthcare provider should check any unusual vaginal bleeding to find out the cause. -
have any other medical conditions
Your healthcare provider may need to check you more carefully if you have certain conditions, such as asthma (wheezing), epilepsy (seizures), diabetes, migraine, endometriosis, lupus, angioedema (swelling of face and tongue), or problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood. -
are going to have surgery or will be on bed rest
Your healthcare provider will let you know if you need to stop taking norethindrone acetate and ethinyl estradiol tablets. -
are breastfeeding
The hormones in norethindrone acetate and ethinyl estradiol tablets can pass into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Some medicines may affect how norethindrone acetate and ethinyl estradiol tablets work. Norethindrone acetate and ethinyl estradiol tablets may also affect how your other medicines work.
Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take norethindrone acetate and ethinyl estradiol tablets?
- Take norethindrone acetate and ethinyl estradiol tablets exactly as your healthcare provider tells you to take it.
- Take 1 norethindrone acetate and ethinyl estradiol tablet at the same time each day.
- You and your healthcare provider should talk regularly (every 3 to 6 months) about the dose you are taking and whether or not you still need treatment with norethindrone acetate and ethinyl estradiol tablets.
What are the possible side effects of norethindrone acetate and ethinyl estradiol tablets?
Side effects are grouped by how serious they are and how often they happen when you are treated.
Serious, but less common side effects include:
- heart attack
- stroke
- blood clots
- dementia
- breast cancer
- cancer of the lining of the uterus (womb)
- cancer of the ovary
- high blood pressure
- high blood sugar
- gallbladder disease
- liver problems
- changes in your thyroid hormone levels
- enlargement of benign tumors of the uterus ("fibroids")
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- new breast lumps
- unusual vaginal bleeding
- changes in vision or speech
- sudden new severe headaches
- severe pains in your chest or legs with or without shortness of breath, weakness and fatigue
Less serious, but common side effects include:
- headache
- breast pain
- irregular vaginal bleeding or spotting
- stomach or abdominal cramps, bloating
- hair loss
- fluid retention
- vaginal yeast infection
These are not all the possible side effects of norethindrone acetate and ethinyl estradiol tablets. For more information, ask your healthcare provider or pharmacist. Tell your healthcare provider if you have any side effects that bother you or does not go away.
To report SUSPECTED ADVERSE REACTIONS, contact Nivagen Pharmaceuticals Toll-free at 1-877-977-0687 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
What can I do to lower my chances of a serious side effect with norethindrone acetate and ethinyl estradiol tablets?
- Talk with your healthcare provider regularly about whether you should continue taking norethindrone acetate and ethinyl estradiol tablets.
- If you have a uterus, talk with your healthcare provider about whether the addition of a progestin is right for you.
- The addition of a progestin is generally recommended for a woman with a uterus to reduce the chance of getting cancer of the uterus (womb).
- See your healthcare provider right away if you develop vaginal bleeding while taking norethindrone acetate and ethinyl estradiol tablets.
- Have a pelvic exam, breast exam and mammogram (breast x-ray) every year unless your healthcare provider tells you something else.
- If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram (breast x-ray), you may need to have breast exams more often.
- If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or use tobacco, you may have a higher chance for getting heart disease.
Ask your healthcare provider for ways to lower your chances of getting heart disease.
How should I store norethindrone acetate and ethinyl estradiol tablets?
- Store norethindrone acetate and ethinyl estradiol tablets at room temperature between 68° to 77° F (20° to 25° C).
Keep norethindrone acetate and ethinyl estradiol tablets out of the reach of children.
General information about the safe and effective use of norethindrone acetate and ethinyl estradiol tablets
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not take norethindrone acetate and ethinyl estradiol tablets for conditions for which it was not prescribed.
Do not give norethindrone acetate and ethinyl estradiol tablets to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about norethindrone acetate and ethinyl estradiol tablets. If you would like more information, talk with your healthcare provider or pharmacist. You can ask your pharmacist or healthcare provider for information about norethindrone acetate and ethinyl estradiol tablets that is written for health professionals.
For more information call 1-877-977-0687.
What are the ingredients in norethindrone acetate and ethinyl estradiol tablets?
Active Ingredients: norethindrone acetate and ethinyl estradiol
Inactive Ingredients: Each white tablet also contains lactose monohydrate, pregelatinized starch, polyethylene glycol, magnesium stearate, ethyl cellulose, vitamin E, titanium dioxide, hypromelloses, macrogol/PEG, triacetin, polydextrose.
Each light yellow tablet also contains lactose monohydrate, pregelatinized starch, polyethylene glycol, magnesium stearate, ethyl cellulose, vitamin E, iron oxide yellow, iron oxide black, talc, polyvinyl alcohol, titanium dioxide, lecithin (soya).
This Patient Information has been approved by the U.S Food and Drug Administration.
Distributed by:
Nivagen Pharmaceuticals, Inc.
Sacramento, CA 95827, USA
Toll Free 1-877-977-0687Manufactured by:
Novast Laboratories Ltd.
Nantong, China 226009I0058
Iss. 08/2022 Rev D -
PATIENT PACKAGE INSERT
FDA-Approved Patient Labeling
Patient Information
Norethindrone Acetate and
Ethinyl Estradiol Tablets, USP
(0.5 mg/2.5 mcg and 1 mg/5 mcg)Read this Patient Information before you start taking norethindrone acetate and ethinyl estradiol tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your menopausal symptoms or your treatment.
What is the most important information I should know about norethindrone acetate and ethinyl estradiol tablets (a combination of estrogen and progestin)? - Do not use estrogens with progestins to prevent heart disease, heart attacks, strokes or dementia (decline of brain function).
- Using estrogens with progestins may increase your chances of getting a heart attack, strokes, breast cancer, or blood clots.
- Using estrogens with progestins may increase your chance of getting dementia, based on a study of women 65 years of age or older.
- Do not use estrogen-alone to prevent heart disease, heart attacks, strokes or dementia.
- Using estrogen-alone may increase your chance of getting cancer of the uterus (womb).
- Using estrogen-alone may increase your chances of getting strokes or blood clots.
- Using estrogen-alone may increase your chance of getting dementia, based on a study of women 65 years of age or older.
- You and your healthcare provider should talk regularly about whether you still need treatment with norethindrone acetate and ethinyl estradiol tablets.
What are norethindrone acetate and ethinyl estradiol tablets?
Norethindrone acetate and ethinyl estradiol tablets are a prescription medicine that contains two kinds of hormones, an estrogen and a progestin.
What are norethindrone acetate and ethinyl estradiol tablets used for?
Norethindrone acetate and ethinyl estradiol tablets are used after menopause to:
-
Reduce moderate to severe hot flushes
Estrogens are hormones made by a woman's ovaries. The ovaries normally stop making estrogens when a woman is between 45 and 55 years old. This drop in body estrogen levels causes the "change of life" or menopause, the end of monthly menstrual periods. Sometimes both ovaries are removed during an operation before natural menopause takes place. The sudden drop in estrogen levels causes "surgical menopause".
When estrogen levels begin dropping, some women get very uncomfortable symptoms, such as feelings of warmth in the face, neck, and chest, or sudden intense episodes of heat and sweating ("hot flashes" or "hot flushes"). In some women the symptoms are mild, and they will not need to take estrogens. In other women, symptoms can be more severe. -
Help reduce your chances of getting osteoporosis (thin weak bones)
If you use norethindrone acetate and ethinyl estradiol tablets only to prevent osteoporosis from menopause, talk with your healthcare provider about whether a different treatment or medicine without estrogens might be better for you. You and your healthcare provider should talk regularly about whether you still need treatment with norethindrone acetate and ethinyl estradiol tablets.
Who should not take norethindrone acetate and ethinyl estradiol tablets?
Do not take norethindrone acetate and ethinyl estradiol tablets if you have had your uterus (womb) removed (hysterectomy).
Norethindrone acetate and ethinyl estradiol tablets contain a progestin to decrease the chance of getting cancer of the uterus. If you do not have a uterus, you do not need a progestin and you should not take norethindrone acetate and ethinyl estradiol tablets.
Do not take norethindrone acetate and ethinyl estradiol tablets if you:
-
have unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb).
Your healthcare provider should check any unusual vaginal bleeding to find out the cause. -
currently have or have had certain cancers.
Estrogens may increase the chance of getting certain types of cancers, including cancer of the breast or uterus. If you have or have had cancer, talk with your healthcare provider about whether you should take norethindrone acetate and ethinyl estradiol tablets - had a stroke or heart attack
- currently have or have had blood clots
- currently have or have had liver problems
- have been diagnosed with a bleeding disorder
-
are allergic to norethindrone acetate and ethinyl estradiol tablets or any of its ingredients.
See the list of ingredients in norethindrone acetate and ethinyl estradiol tablets at the end of this leaflet. -
think you may be pregnant
Norethindrone acetate and ethinyl estradiol tablets are not for pregnant women. If you think you may be pregnant, you should have a pregnancy test and know the results. Do not take norethindrone acetate and ethinyl estradiol tablets if the test is positive and talk to your healthcare provider.
What should I tell my healthcare provider before I take norethindrone acetate and ethinyl estradiol tablets?
Before you take norethindrone acetate and ethinyl estradiol tablets, tell your healthcare provider if you:
-
have any unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb).
Your healthcare provider should check any unusual vaginal bleeding to find out the cause. -
have any other medical conditions
Your healthcare provider may need to check you more carefully if you have certain conditions, such as asthma (wheezing), epilepsy (seizures), diabetes, migraine, endometriosis, lupus, angioedema (swelling of face and tongue), or problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood. -
are going to have surgery or will be on bed rest
Your healthcare provider will let you know if you need to stop taking norethindrone acetate and ethinyl estradiol tablets. -
are breastfeeding
The hormones in norethindrone acetate and ethinyl estradiol tablets can pass into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Some medicines may affect how norethindrone acetate and ethinyl estradiol tablets work. Norethindrone acetate and ethinyl estradiol tablets may also affect how your other medicines work.
Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take norethindrone acetate and ethinyl estradiol tablets?
- Take norethindrone acetate and ethinyl estradiol tablets exactly as your healthcare provider tells you to take it.
- Take 1 norethindrone acetate and ethinyl estradiol tablet at the same time each day.
- You and your healthcare provider should talk regularly (every 3 to 6 months) about the dose you are taking and whether or not you still need treatment with norethindrone acetate and ethinyl estradiol tablets.
What are the possible side effects of norethindrone acetate and ethinyl estradiol tablets?
Side effects are grouped by how serious they are and how often they happen when you are treated.
Serious, but less common side effects include:
- heart attack
- stroke
- blood clots
- dementia
- breast cancer
- cancer of the lining of the uterus (womb)
- cancer of the ovary
- high blood pressure
- high blood sugar
- gallbladder disease
- liver problems
- changes in your thyroid hormone levels
- enlargement of benign tumors of the uterus ("fibroids")
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- new breast lumps
- unusual vaginal bleeding
- changes in vision or speech
- sudden new severe headaches
- severe pains in your chest or legs with or without shortness of breath, weakness and fatigue
Less serious, but common side effects include:
- headache
- breast pain
- irregular vaginal bleeding or spotting
- stomach or abdominal cramps, bloating
- hair loss
- fluid retention
- vaginal yeast infection
These are not all the possible side effects of norethindrone acetate and ethinyl estradiol tablets. For more information, ask your healthcare provider or pharmacist. Tell your healthcare provider if you have any side effects that bother you or does not go away.
To report SUSPECTED ADVERSE REACTIONS, contact Nivagen Pharmaceuticals Toll-free at 1-877-977-0687 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
What can I do to lower my chances of a serious side effect with norethindrone acetate and ethinyl estradiol tablets?
- Talk with your healthcare provider regularly about whether you should continue taking norethindrone acetate and ethinyl estradiol tablets.
- If you have a uterus, talk with your healthcare provider about whether the addition of a progestin is right for you.
- The addition of a progestin is generally recommended for a woman with a uterus to reduce the chance of getting cancer of the uterus (womb).
- See your healthcare provider right away if you develop vaginal bleeding while taking norethindrone acetate and ethinyl estradiol tablets.
- Have a pelvic exam, breast exam and mammogram (breast x-ray) every year unless your healthcare provider tells you something else.
- If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram (breast x-ray), you may need to have breast exams more often.
- If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or use tobacco, you may have a higher chance for getting heart disease.
Ask your healthcare provider for ways to lower your chances of getting heart disease.
How should I store norethindrone acetate and ethinyl estradiol tablets?
- Store norethindrone acetate and ethinyl estradiol tablets at room temperature between 68° to 77° F (20° to 25° C).
Keep norethindrone acetate and ethinyl estradiol tablets out of the reach of children.
General information about the safe and effective use of norethindrone acetate and ethinyl estradiol tablets
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not take norethindrone acetate and ethinyl estradiol tablets for conditions for which it was not prescribed.
Do not give norethindrone acetate and ethinyl estradiol tablets to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about norethindrone acetate and ethinyl estradiol tablets. If you would like more information, talk with your healthcare provider or pharmacist. You can ask your pharmacist or healthcare provider for information about norethindrone acetate and ethinyl estradiol tablets that is written for health professionals.
For more information call 1-877-977-0687.
What are the ingredients in norethindrone acetate and ethinyl estradiol tablets?
Active Ingredients: norethindrone acetate and ethinyl estradiol
Inactive Ingredients: Each white tablet also contains lactose monohydrate, pregelatinized starch, polyethylene glycol, magnesium stearate, ethyl cellulose, vitamin E, titanium dioxide, hypromelloses, macrogol/PEG, triacetin, polydextrose.
Each light yellow tablet also contains lactose monohydrate, pregelatinized starch, polyethylene glycol, magnesium stearate, ethyl cellulose, vitamin E, iron oxide yellow, iron oxide black, talc, polyvinyl alcohol, titanium dioxide, lecithin (soya).
This Patient Information has been approved by the U.S Food and Drug Administration.
Distributed by:
Nivagen Pharmaceuticals, Inc.
Sacramento, CA 95827, USA
Toll Free 1-877-977-0687Manufactured by:
Novast Laboratories Ltd.
Nantong, China 226009I 0059
Rx only
Iss. 08/2022 Rev. B -
PRINCIPAL DISPLAY PANEL - 1 mg/5 mcg Tablet Blister Pack Carton
3 Blister Cards of 28 Tablets each.
NDC 75834-130-29Norethindrone Acetate and
Ethinyl Estradiol Tablets, USP1 mg/5 mcg
Each light yellow tablet contains norethindrone acetate 1 mg
and ethinyl estradiol 5 mcg.
Each tablet dispenser contains 28 white tabletsThis product (like all oral contraceptives) is intended to prevent pregnancy.
It does not protect against HIV infection (AIDS) and other sexually
transmitted diseases.Rx Only
NIVAGEN
PHARMACEUTICALS
-
PRINCIPAL DISPLAY PANEL - 0.5 mg/2.5 mcg Tablet Blister Pack Carton
3 Blister Cards of 28 Tablets each.
NDC 75834-129-29Norethindrone Acetate and
Ethinyl Estradiol Tablets, USP0.5 mg/2.5 mcg
Each light yellow tablet contains norethindrone acetate 0.5 mg
and ethinyl estradiol 2.5 mcg.
Each tablet dispenser contains 28 light yellow tabletsThis product (like all oral contraceptives) is intended to prevent pregnancy.
It does not protect against HIV infection (AIDS) and other sexually
transmitted diseases.Rx Only
NIVAGEN
PHARMACEUTICALS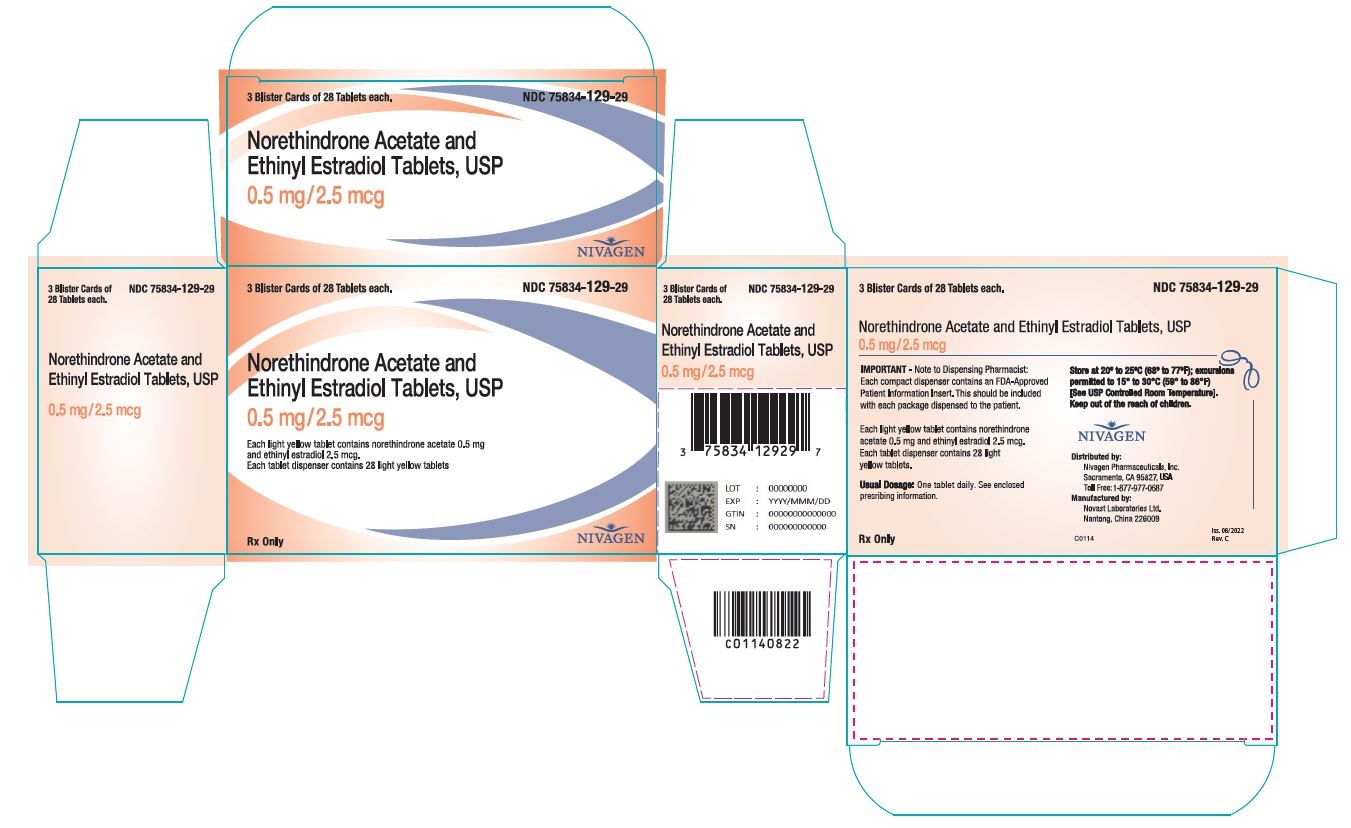
-
INGREDIENTS AND APPEARANCE
NORETHINDRONE ACETATE AND ETHINYL ESTRADIOL
norethindrone acetate and ethinyl estradiol tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75834-130 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Norethindrone Acetate (UNII: 9S44LIC7OJ) (NORETHINDRONE - UNII:T18F433X4S) Norethindrone Acetate 1 mg Ethinyl Estradiol (UNII: 423D2T571U) (Ethinyl Estradiol - UNII:423D2T571U) Ethinyl Estradiol 0.005 mg Inactive Ingredients Ingredient Name Strength Lactose monohydrate (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) Magnesium Stearate (UNII: 70097M6I30) HYDROXYETHYL CELLULOSE (140 MPA.S AT 5%) (UNII: 8136Y38GY5) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) Titanium Dioxide (UNII: 15FIX9V2JP) Hypromellose, Unspecified (UNII: 3NXW29V3WO) Triacetin (UNII: XHX3C3X673) Polydextrose (UNII: VH2XOU12IE) Product Characteristics Color WHITE Score no score Shape ROUND Size 5mm Flavor Imprint Code N2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75834-130-29 3 in 1 CARTON 11/25/2016 1 NDC:75834-130-84 28 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:75834-130-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 11/25/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203435 11/25/2016 NORETHINDRONE ACETATE AND ETHINYL ESTRADIOL
norethindrone acetate and ethinyl estradiol tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75834-129 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Norethindrone Acetate (UNII: 9S44LIC7OJ) (NORETHINDRONE - UNII:T18F433X4S) Norethindrone Acetate 0.5 mg Ethinyl Estradiol (UNII: 423D2T571U) (Ethinyl Estradiol - UNII:423D2T571U) Ethinyl Estradiol 0.0025 mg Inactive Ingredients Ingredient Name Strength Lactose monohydrate (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) Magnesium Stearate (UNII: 70097M6I30) HYDROXYETHYL CELLULOSE (140 MPA.S AT 5%) (UNII: 8136Y38GY5) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Talc (UNII: 7SEV7J4R1U) Polyvinyl Alcohol, Unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) Product Characteristics Color YELLOW (Light) Score no score Shape ROUND Size 5mm Flavor Imprint Code N1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75834-129-29 3 in 1 CARTON 11/25/2016 1 NDC:75834-129-84 28 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:75834-129-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 11/25/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203435 11/25/2016 Labeler - Nivagen Pharmaceuticals, Inc. (052032418) Registrant - Novast Laboratories, Ltd. (527695995) Establishment Name Address ID/FEI Business Operations Novast Laboratories, Ltd. 527695995 analysis(75834-130, 75834-129) , label(75834-130, 75834-129) , manufacture(75834-130, 75834-129) , pack(75834-130, 75834-129)