Label: CONTROLLING BALM WITH TEA TREE OIL- salicylic acid emulsion
- NDC Code(s): 35192-016-05
- Packager: CA-BOTANA INTERNATIONAL
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
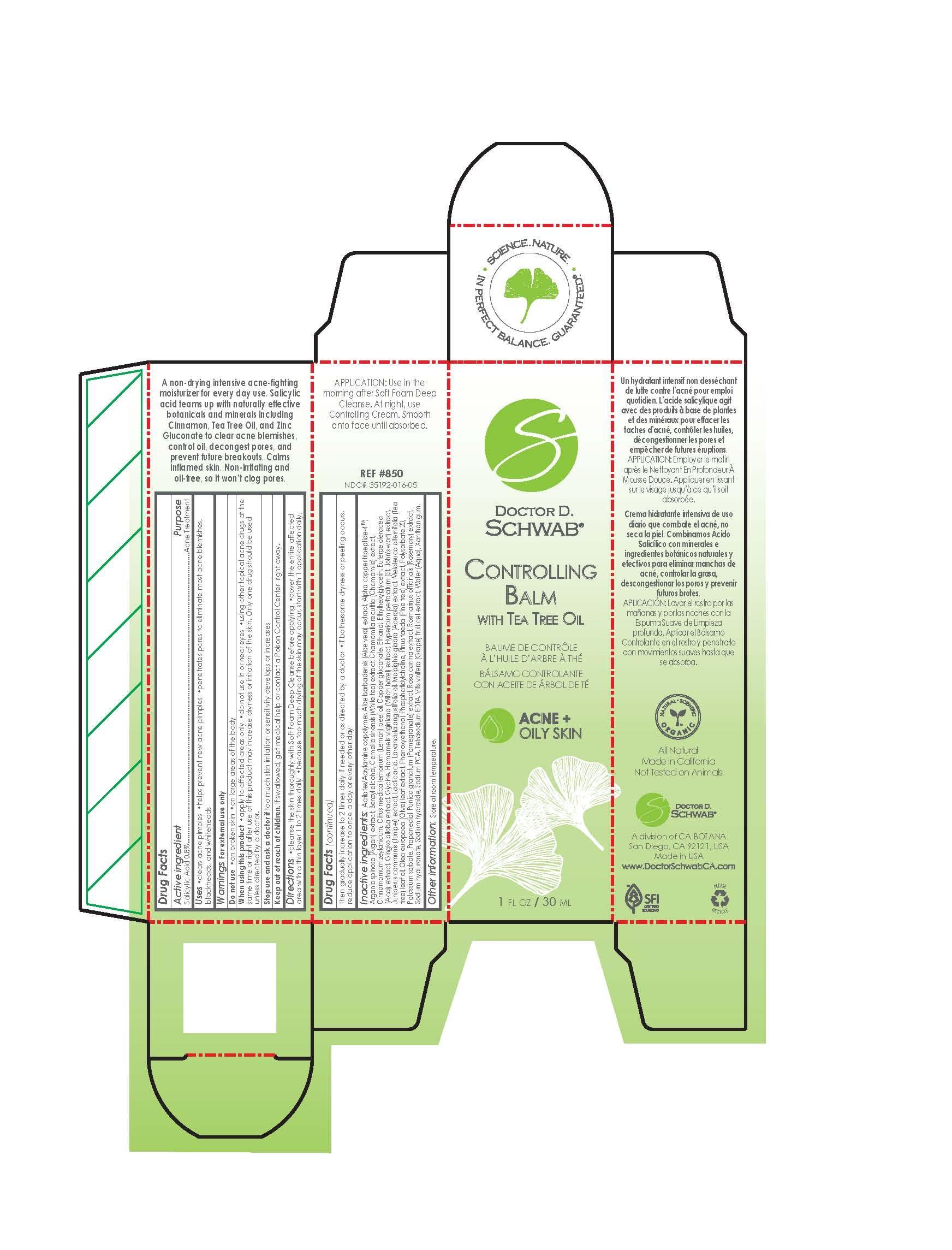
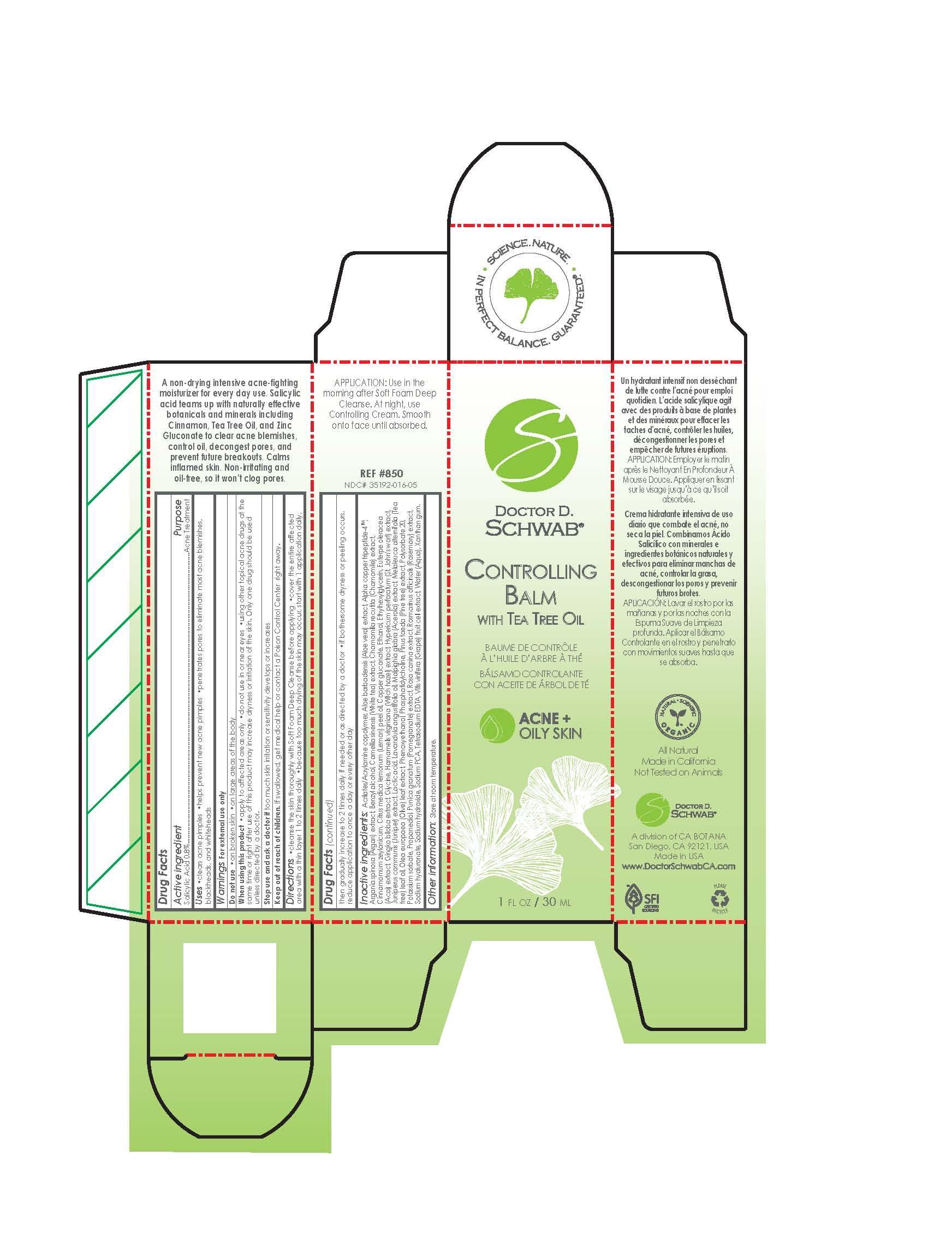
WARNINGS
Warnings:
For external use only.
Do not use on wounds or damaged skin
When using this product: use only as directed. Avoid contact with eyes. Do no bandage tightly
Stop use and ask a doctor if: redness is present. Irritation develops. Condition worsens or symptoms persist more than 7 days. Symptoms clear up and occur again within a few days.
Store at room temperature. Lot number and expiration date see crimp or see box. -
DOSAGE & ADMINISTRATION
cleanse the skin thoroughly with soft foam deep cleanse before applying. cover the entire affected aread with a thin layer 1 to 2 times daily. because too much drying of the skin may occure, start with 1 application daily, then gradually increase to 2 times daily if needed or as directed by a doctor. if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
INACTIVE INGREDIENT
Acrilates/Acrylamine Copolymer Aloe barbadensis (Aloe vera) extract Alpha Copper Tripeptide-4 TM Argania Spinosa (Argan) extract Benzyl Alcohol Camellia sinensis (White tea) extract Chamomilla recutita (Chamomile) extract Cinnamomum Zeylanicum Citrus medica lemonum (Lemon)Peel Oil Copper Gluconate Ethanol Ethylhexylglycerin Euterpe oleracea (Acai) extract Gingko Biloba extract Glycine Hamamelis virginiana (Witchhazel) extract Hypericum perforatum (St John's wort) extract Juniperus communis (Juniper) extract Lactic acid Lavandula angustifolia oil Malpighia glabra (Acerola) extract Melaleuca Alternifolia (Tea tree) leaf oil Olea europaea (Olive) leaf extract Phenoxyethanol Phosphatidylcholine Pinus Taeda (Pine tree) extract Polysorbate 20 Potassium Sorbate Propanediol Punica granatum (Pomegranate) extract Rosa canina extract Rosmarinus officinalis (Rosemary) extract Sodium Hyaluronate Sodium Hydroxide Sodium PC Tetra sodium Vitis Vinifera ( Grape) extract Water (Aqua) Xanthan Gum - ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- PURPOSE
- PRINCIPAL DISPLAY PANEL
- Package Label Display Panel
-
INGREDIENTS AND APPEARANCE
CONTROLLING BALM WITH TEA TREE OIL
salicylic acid emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:35192-016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.24 mg in 29.6 mg Inactive Ingredients Ingredient Name Strength ROSA CANINA FLOWER (UNII: 81MCR2UQ6Q) ROSEMARY (UNII: IJ67X351P9) SODIUM HYDROXIDE (UNII: 55X04QC32I) VITIS VINIFERA FLOWERING TOP (UNII: 6TG3V35HTV) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PROPYLENE GLYCOL, (R)- (UNII: 602HN5L69H) 1,2-DIOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE (UNII: H026DM5V6U) GLATIRAMER ACETATE (UNII: 5M691HL4BO) ALOE VERA LEAF (UNII: ZY81Z83H0X) COPPER (UNII: 789U1901C5) ARGANIA SPINOSA LEAF (UNII: 51XV5WTF7E) .ALPHA.,.ALPHA.-DIMETHYLBENZYL ALCOHOL (UNII: JE030BGE05) CAMELLIA SINENSIS FLOWER (UNII: 9I2BJY2J17) CHAMOMILE (UNII: FGL3685T2X) CINNAMON (UNII: 5S29HWU6QB) LEMON OIL (UNII: I9GRO824LL) COPPER GLUCONATE (UNII: RV823G6G67) ALCOHOL (UNII: 3K9958V90M) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ACAI (UNII: 46AM2VJ0AW) GINKGO (UNII: 19FUJ2C58T) GLYCINE (UNII: TE7660XO1C) HAMAMELIS VIRGINIANA BARK (UNII: IH3063S9MY) HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) JUNIPER BERRY OIL (UNII: SZH16H44UY) LACTIC ACID (UNII: 33X04XA5AT) LAVENDER OIL (UNII: ZBP1YXW0H8) MALPIGHIA EMARGINATA SEED (UNII: 1X7L93686M) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) PHENOXYETHANOL (UNII: HIE492ZZ3T) PINUS TAEDA BARK (UNII: 9I1F994SVG) POLYSORBATE 20 (UNII: 7T1F30V5YH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PUNICA GRANATUM FLOWER (UNII: D9B634V4GP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35192-016-05 29.4 mg in 1 TUBE; Type 0: Not a Combination Product 11/02/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/09/2014 Labeler - CA-BOTANA INTERNATIONAL (106276728) Registrant - RODOLFO UGELSTAD (106276728) Establishment Name Address ID/FEI Business Operations CA-BOTANA INTERNATIONAL 106276728 manufacture(35192-016)