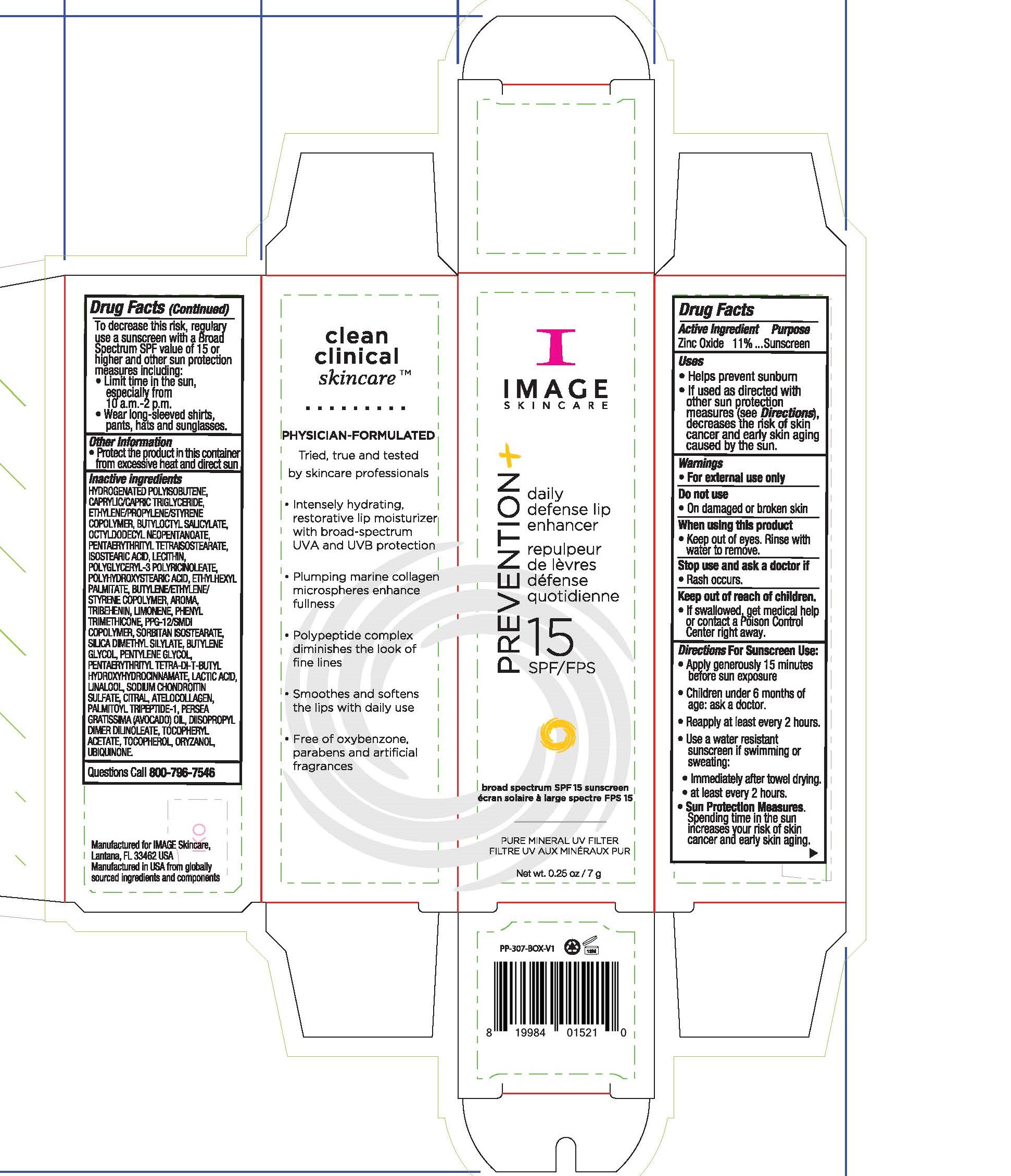
Label: IMAGE SKINCARE PREVENTION PLUS DAILY DEFENSE LIP ENHANCER- zinc oxide lip sunscreen cream
- NDC Code(s): 60232-0027-0
- Packager: Swiss-American CDMO, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Warnings
- Keep out of Reach of Children
- Active Ingredients
- Uses
- Uses
-
Directions For Sunscreen Use
Apply generously 15 minutes before sun exposure. Children under 6 months of age: ask a doctor. Reapply at least every 2 hours. Use a water resistant sunscreen if swimming or sweating. Immediately after towel drying. At least every 2 hours. Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectgrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10am to 2pm, wear long-sleeved shirts, pants, hats and sunglasses.
- Other Information
-
Inactive Ingredients
hydrogenated polyisobutene, caprylic/capric triglyceride, ethylene/propylene/styrene copolymer, butyloctyl salicylate, octyldodecyl neopentanoate, pentaerythrityl tetraisostearate, isostearic acid, lecithin, polyglyceryl-3 polyricnoleate, polyhydroxystearic acid, ethylhexyl palmitate, bytlene/ethylene/styrene copolymer, aroma, tribehenin, limonene, phenyl trimethicone, ppg-12/smdi copolymer, sorbitan isostearate, silica dimethyl silylate, butylene glycol, pentylene glycol, pentaerythrityl tetra-di-t-butyl hydroxylhydrocinnamate, lacic acid, linalool, sodium chondroitin sulfate, citral, atelocollagen, palmitoyl tripeptide-1, persea gratissma (avocado) oil, diisopropyl dimer dilinoleate, tocopheryl acetate, tocopherol, oryzanol, ubiquinone
- Questions
- Labeling
-
INGREDIENTS AND APPEARANCE
IMAGE SKINCARE PREVENTION PLUS DAILY DEFENSE LIP ENHANCER
zinc oxide lip sunscreen creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60232-0027 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 110 g in 1000 g Inactive Ingredients Ingredient Name Strength OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) AVOCADO OIL (UNII: 6VNO72PFC1) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PENTAERYTHRITYL TETRAISOSTEARATE (UNII: 9D7IK5483F) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PENTYLENE GLYCOL (UNII: 50C1307PZG) SODIUM CHONDROITIN SULFATE (PORCINE; 5500 MW) (UNII: H5BJH23Z9A) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) LACTIC ACID (UNII: 33X04XA5AT) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) ORYZANOL (UNII: SST9XCL51M) UBIDECARENONE (UNII: EJ27X76M46) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) LIMONENE, (+/-)- (UNII: 9MC3I34447) LINALOOL, (+/-)- (UNII: D81QY6I88E) CITRAL (UNII: T7EU0O9VPP) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60232-0027-0 7 g in 1 TUBE; Type 0: Not a Combination Product 01/02/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/02/2020 Labeler - Swiss-American CDMO, LLC (080170933) Registrant - Swiss-American CDMO, LLC (080170933) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 manufacture(60232-0027)