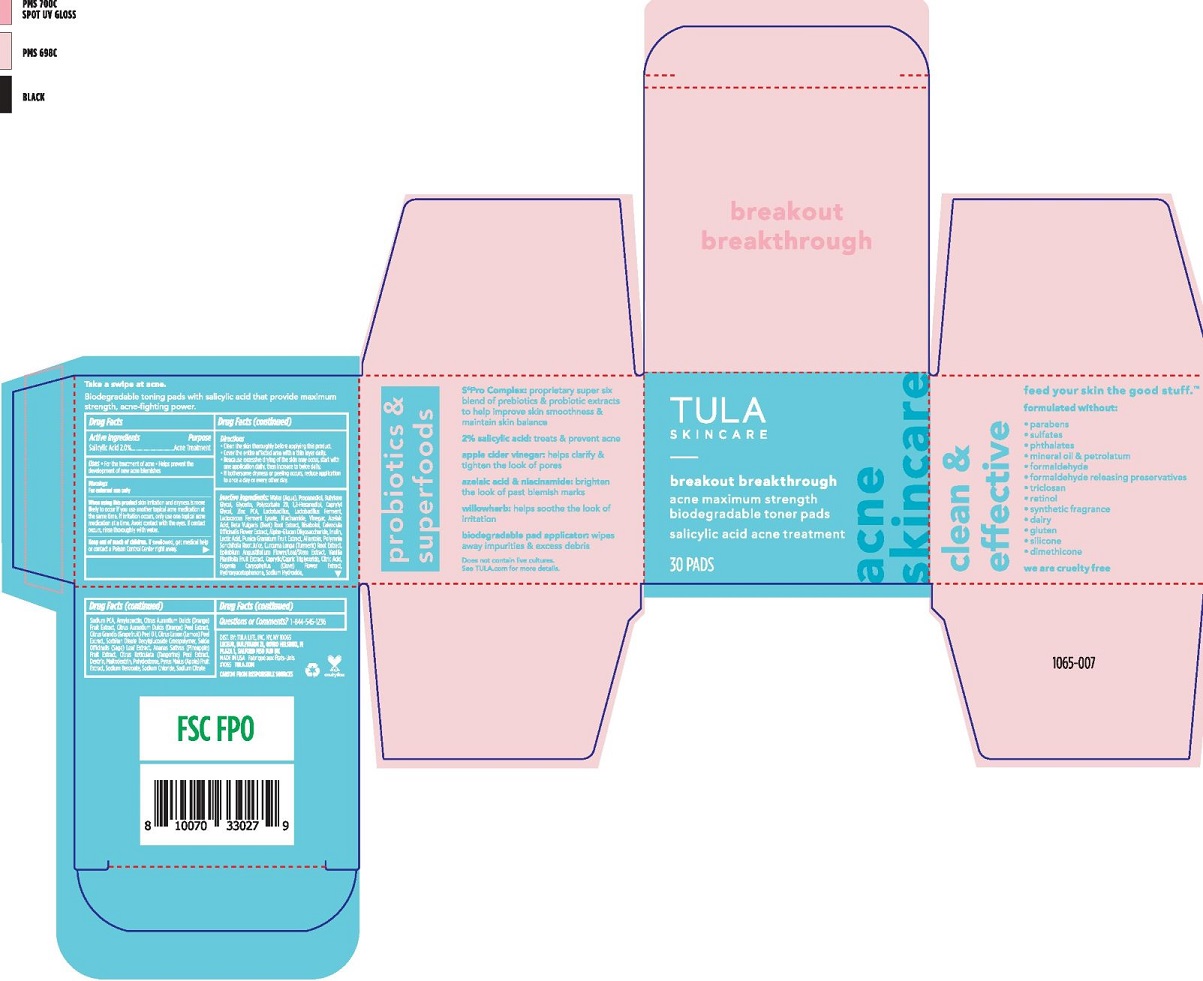
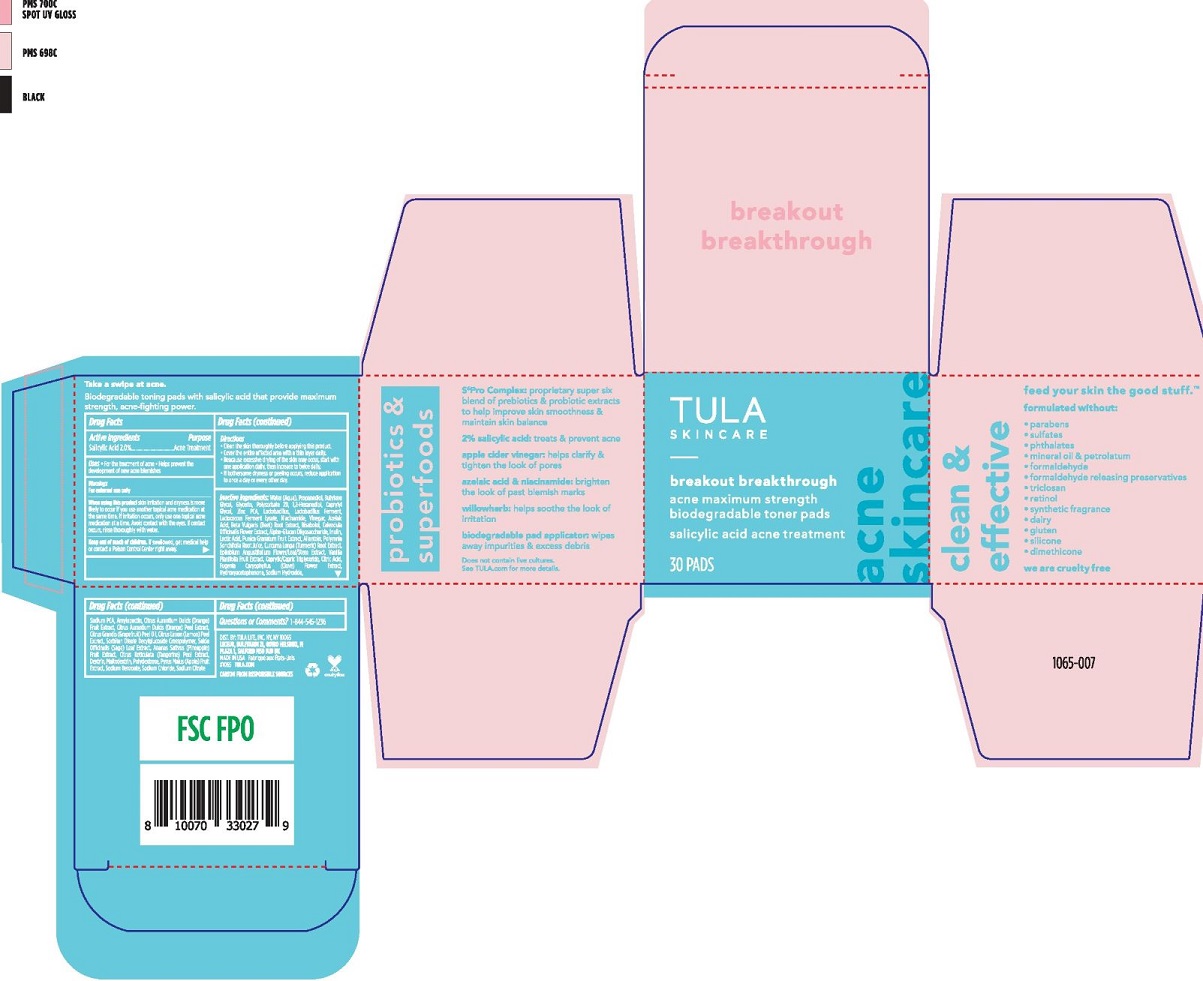
Label: TULA SKINCARE BREAKOUT BREAKTHROUGH ACNE TONER PADS- salicylic acid swab swab
- NDC Code(s): 72296-070-01
- Packager: Tula Life LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only
When using this product • skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time. • Avoid contact with the eyes. If contact occurs, rinse thoroughly with water. - Keep out of reach of children
-
Directions
• Clean the skin thoroughly before applying this
product.
• Cover the entire affected area with a thin layer daily.
• Because excessive drying of the skin may occur,
start with one application daily, then increase to
twice daily.
• If bothersome dryness or peeling occurs, reduce
application to once a day or every other day. -
Inactive ingredients
WATER (AQUA), PROPANEDIOL, BUTYLENE GLYCOL, GLYCERIN, POLYSORBATE 20, 1,2-HEXANEDIOL, CAPRYLYL GLYCOL, ZINC PCA, DEXTRIN, POLYDEXTROSE, HYDROXYACETOPHENONE, NIACINAMIDE, AMYLOPECTIN, SODIUM CITRATE, SODIUM PCA, VINEGAR, LACTOCOCCUS FERMENT LYSATE, SORBITAN OLEATE DECYLGLUCOSIDE CROSSPOLYMER, EPILOBIUM ANGUSTIFOLIUM FLOWER/LEAF/STEM EXTRACT, ALLANTOIN, AZELAIC ACID, CURCUMA LONGA (TURMERIC) ROOT EXTRACT, ALPHA-GLUCAN OLIGOSACCHARIDE, BISABOLOL, PYRUS MALUS (APPLE) FRUIT EXTRACT, CAPRYLIC/CAPRIC TRIGLYCERIDE, PUNICA GRANATUM FRUIT EXTRACT, BETA VULGARIS (BEET) ROOT EXTRACT, POLYMNIA SONCHIFOLIA ROOT JUICE, CITRUS GRANDIS (GRAPEFRUIT) PEEL OIL, CITRUS AURANTIUM DULCIS (ORANGE) PEEL EXTRACT, LACTOBACILLUS FERMENT, INULIN, MALTODEXTRIN, CITRUS LIMON (LEMON) PEEL EXTRACT, CALENDULA OFFICINALIS FLOWER EXTRACT, CITRUS RETICULATA (TANGERINE) PEEL EXTRACT, LACTOBACILLUS, VANILLA PLANIFOLIA FRUIT EXTRACT, ANANAS SATIVUS (PINEAPPLE) FRUIT EXTRACT, CITRUS AURANTIUM DULCIS (ORANGE) FRUIT EXTRACT, EUGENIA CARYOPHYLLUS (CLOVE) FLOWER EXTRACT, SALVIA OFFICINALIS (SAGE) LEAF EXTRACT, CITRIC ACID, SODIUM HYDROXIDE, LACTIC ACID, SODIUM BENZOATE, SODIUM CHLORIDE
- Questions or Comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TULA SKINCARE BREAKOUT BREAKTHROUGH ACNE TONER PADS
salicylic acid swab swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72296-070 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.02 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLYSORBATE 20 (UNII: 7T1F30V5YH) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) NIACINAMIDE (UNII: 25X51I8RD4) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LACTIC ACID (UNII: 33X04XA5AT) AZELAIC ACID (UNII: F2VW3D43YT) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PYRROLIDONE CARBOXYLIC ACID (UNII: 6VT1YZM21H) MALTODEXTRIN (UNII: 7CVR7L4A2D) POLYDEXTROSE (UNII: VH2XOU12IE) AMYLOPECTIN, UNSPECIFIED SOURCE (UNII: 4XO4QFV777) SODIUM CITRATE (UNII: 1Q73Q2JULR) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) APPLE CIDER VINEGAR (UNII: 0UE22Q87VC) LACTOCOCCUS LACTIS (UNII: F1A0PSN10V) EPILOBIUM ANGUSTIFOLIUM WHOLE (UNII: C278QS9YBT) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM BENZOATE (UNII: OJ245FE5EU) ALLANTOIN (UNII: 344S277G0Z) CURCUMA LONGA WHOLE (UNII: W5488JUO8U) .ALPHA.-GLUCAN OLIGOSACCHARIDE (UNII: S95658MI3W) APPLE JUICE (UNII: 9871T0PD5P) CAPRYLIC/CAPRIC MONO/DI-GLYCERIDES (UNII: U72Q2I8C85) PUNICA GRANATUM SEED (UNII: 7294Z34NS7) BEET JUICE (UNII: IOZ32L9H3O) CITRUS AURANTIUM FRUIT RIND (UNII: 055456JHI7) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) LACTOBACILLUS FERMENTUM (UNII: 2C1F12C6AP) INULIN (UNII: JOS53KRJ01) CITRUS LIMON FRUIT OIL (UNII: 0HNC1J1YED) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CITRUS RETICULATA FRUIT OIL (UNII: 25P9H3QU5E) VANILLA PLANIFOLIA SEED (UNII: GKQ4MH0F2E) PINEAPPLE (UNII: 2A88ZO081O) ORANGE JUICE (UNII: 5A9KE2L9L3) CLOVE (UNII: K48IKT5321) SAGE (UNII: 065C5D077J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72296-070-01 1 in 1 CARTON 02/03/2022 1 30 in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 02/03/2022 Labeler - Tula Life LLC (080051358)