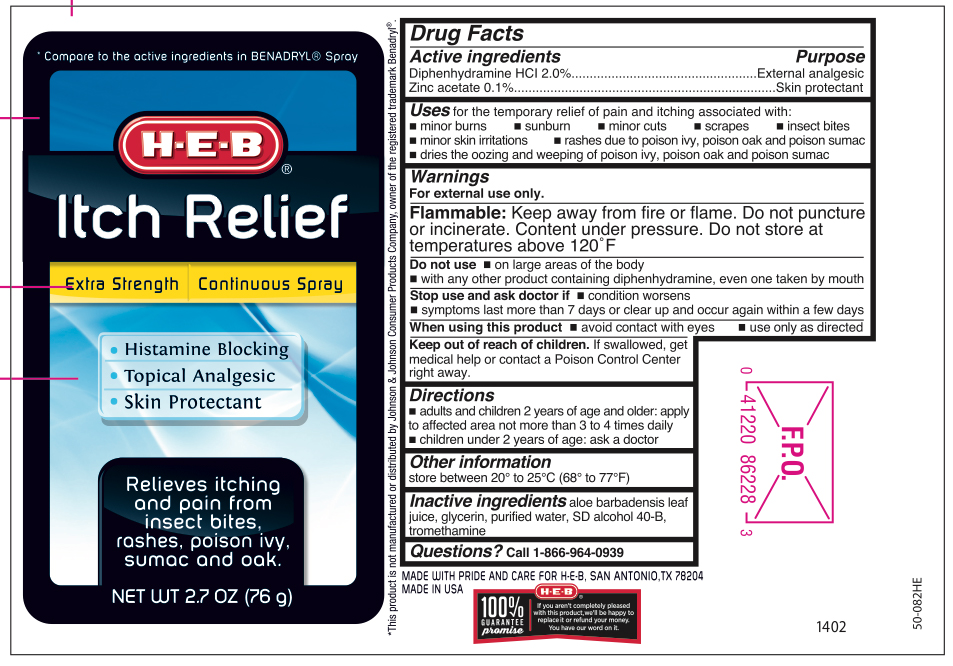
Label: DIPHENHYDRAMINE HCL AND ZINC ACETATE- extra strength itch relief spray
- NDC Code(s): 37808-526-27
- Packager: HEB
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only.
Flammable:
Keep away from fire or flame. Do not puncture or incinerate. Contents under pressure. Do not store at temperature above 120ºF. Intentional misuse by deliberately concentrating and inhaling contents cans be harmful or fatal.
Do not use
- on large areas of the body
- with any other products containing diphenhydramine, even one taken by mouth
- Directions
- Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HCL AND ZINC ACETATE
extra strength itch relief sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37808-526 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 1.52 g in 76 g ZINC ACETATE (UNII: FM5526K07A) (ZINC CATION - UNII:13S1S8SF37) ZINC ACETATE 0.076 g in 76 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) TROMETHAMINE (UNII: 023C2WHX2V) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37808-526-27 76 g in 1 CAN; Type 0: Not a Combination Product 12/22/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/22/2017 Labeler - HEB (007924756)