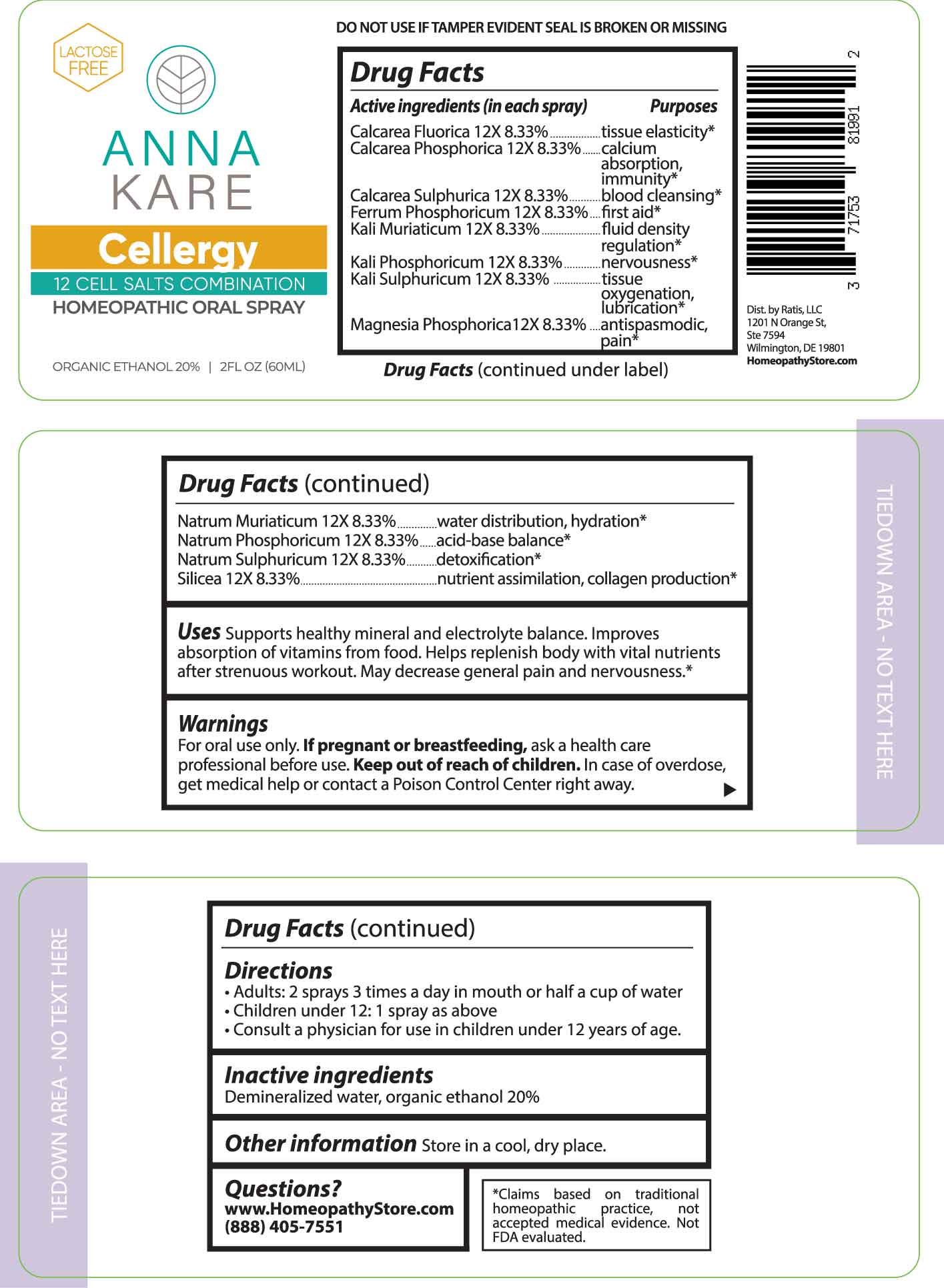
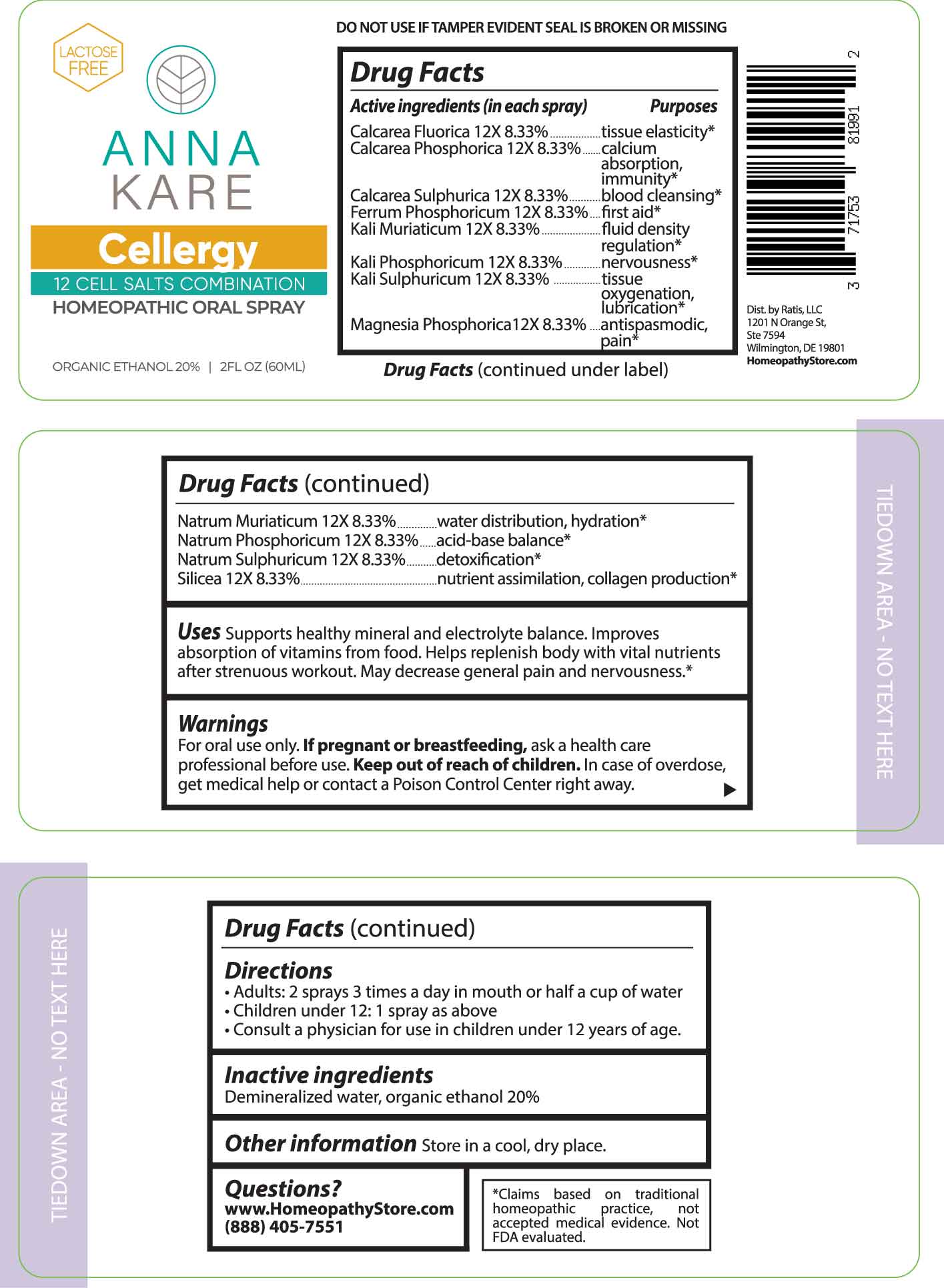
Label: CELLERGY- calcarea fluorica, calcarea phosphorica, calcarea sulphurica, ferrum phosphoricum, kali muriaticum, kali phosphoricum, kali sulphuricum, magnesia phosphorica, natrum muriaticum, natrum phosphoricum, natrum sulphuricum, silicea. spray
- NDC Code(s): 71753-8199-1
- Packager: Ratis, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS:
(in each spray) Calcarea Fluorica 12X 8.33%, Calcarea Phosphorica 12X 8.33%, Calcarea Sulphurica 12X 8.33%, Ferrum Phosphoricum 12X 8.33%, Kali Muriaticum 12X 8.33%, Kali Phosphoricum 12X 8.33%, Kali Sulphuricum 12X 8.33%, Magnesia Phosphorica 12X 8.33%, Natrum Muriaticum 12X 8.33%, Natrum Phosphoricum 12X 8.33%, Natrum Sulphuricum 12X 8.33%, Silicea 12X 8.33%.
-
PURPOSES:
Calcarea Fluorica - tissue elasticity,* Calcarea Phosphorica – calcium absorption, Immunity,* Calcarea Sulphurica – blood cleansing,* Ferrum Phosphoricum – first aid,* Kali Muriaticum – fluid density regulation,* Kali Phosphoricum - nervousness,* Kali Sulphuricum – tissue oxygenation, lubrication,* Magnesia Phosphorica – antispasmodic, pain,* Natrum Muriaticum – water distribution, hydration,* Natrum Phosphoricum – acid-base balance,* Natrum Sulphuricum - detoxification,* Silicea – nutrient assimilation, collagen production.*
-
USES:
Supports healthy mineral and electrolyte balance. Improves absorption of vitamins from food. Helps replenish body with vital nutrients after strenuous workout. May decrease general pain and nervousness.*
*Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABLE DISPLAY:
-
INGREDIENTS AND APPEARANCE
CELLERGY
calcarea fluorica, calcarea phosphorica, calcarea sulphurica, ferrum phosphoricum, kali muriaticum, kali phosphoricum, kali sulphuricum, magnesia phosphorica, natrum muriaticum, natrum phosphoricum, natrum sulphuricum, silicea. sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71753-8199 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 12 [hp_X] in 1 mL TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 12 [hp_X] in 1 mL CALCIUM SULFATE ANHYDROUS (UNII: E934B3V59H) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFATE ANHYDROUS 12 [hp_X] in 1 mL FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 12 [hp_X] in 1 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CATION 12 [hp_X] in 1 mL DIBASIC POTASSIUM PHOSPHATE (UNII: CI71S98N1Z) (PHOSPHATE ION - UNII:NK08V8K8HR) DIBASIC POTASSIUM PHOSPHATE 12 [hp_X] in 1 mL POTASSIUM SULFATE (UNII: 1K573LC5TV) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM SULFATE 12 [hp_X] in 1 mL MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 12 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 12 [hp_X] in 1 mL SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE 12 [hp_X] in 1 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 12 [hp_X] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71753-8199-1 60 mL in 1 BOTTLE, SPRAY; Type 1: Convenience Kit of Co-Package 08/07/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/07/2023 Labeler - Ratis, LLC (964594324)