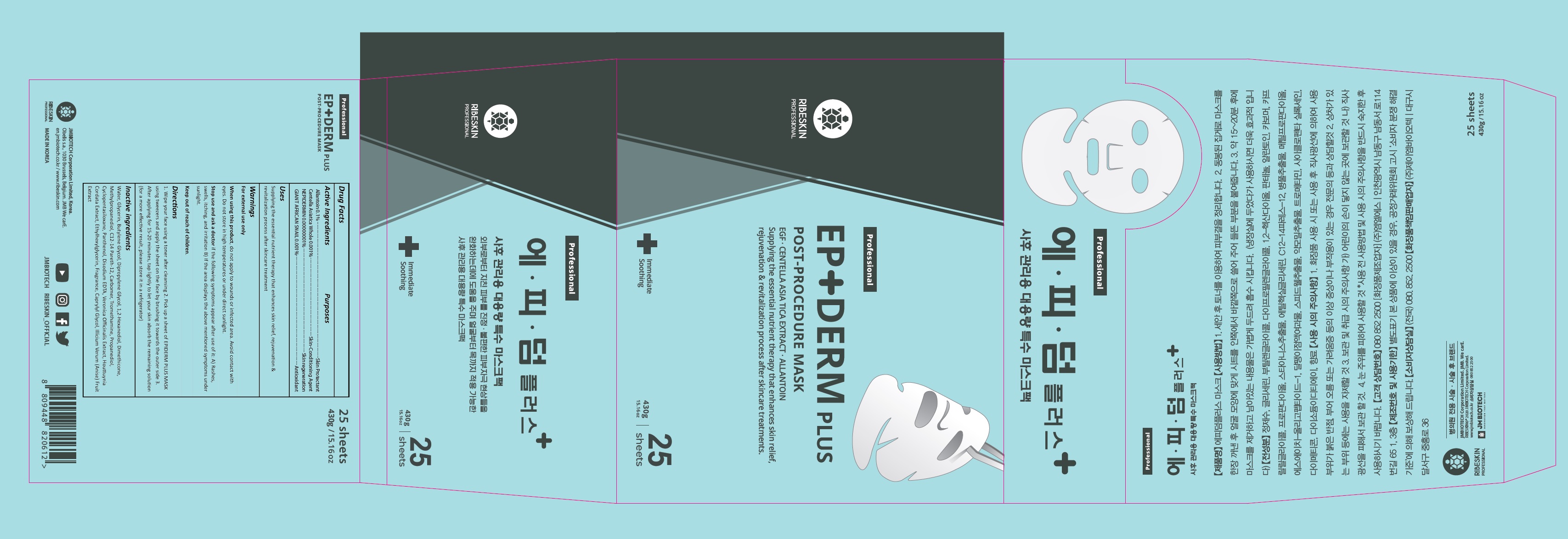
Label: RIBESKIN EPIDERM PLUS MASK- allantoin, centella asiatica whole, nepidermin, giant african snail cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 82224-001-01 - Packager: JM Bio-Tech Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 27, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
1. Wipe your face using a toner after cleansing 2. Pick up a sheet of EPIDERM PLUS MASK using tweezers and apply the sheet on the face by brushing it towards the outer side 3. After applying for 15-20 minutes, tap lightly to let your skin absorb the remaining solution (for a more effective result, please store it in a refrigerator)
-
WARNINGS
For external use only
When using this product, do not apply to wounds or infected area. Avoid contact with eyes. Do not store in high temperatures or under direct sunlight.
Stop use and ask a doctor if the following symptoms appear after use of it: A) Rashes, swells, itching, and irritation B) If the area displays the above mentioned symptoms under sunlight.
Keep out of reach of children. - KEEP OUT OF REACH OF CHILDREN
-
INACTIVE INGREDIENT
Water, Glycerin, Butylene Glycol, Dipropylene Glycol, 1,2-Hexanediol, Dimethicone, Methylpropanediol, C12-14 Pareth-12, Carbomer, Tromethamine, Propanediol, Cyclopentasiloxane, Panthenol, Disodium EDTA, Snail Secretion Filtrate, Veronica Officinalis Extract, Houttuynia Cordata Extract, Ethylhexylglycerin, Fragrance, Caprylyl Glycol, Illicium Verum (Anise) Fruit Extract, sh-Oligopeptide-1
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RIBESKIN EPIDERM PLUS MASK
allantoin, centella asiatica whole, nepidermin, giant african snail creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82224-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 0.1 g in 100 g CENTELLA ASIATICA WHOLE (UNII: 7M867G6T1U) (CENTELLA ASIATICA - UNII:7M867G6T1U) CENTELLA ASIATICA WHOLE 0.001 g in 100 g NEPIDERMIN (UNII: TZK30RF92W) (HUMAN EPIDERMAL GROWTH FACTOR - UNII:TZK30RF92W) NEPIDERMIN 0.00000001 g in 100 g GIANT AFRICAN SNAIL (UNII: 5A1SK0DCIZ) (GIANT AFRICAN SNAIL - UNII:5A1SK0DCIZ) GIANT AFRICAN SNAIL 0.001 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) DIMETHICONE (UNII: 92RU3N3Y1O) METHYLPROPANEDIOL (UNII: N8F53B3R4R) C12-14 PARETH-12 (UNII: M0LJS773XW) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) TROMETHAMINE (UNII: 023C2WHX2V) PROPANEDIOL (UNII: 5965N8W85T) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PANTHENOL (UNII: WV9CM0O67Z) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) HOUTTUYNIA CORDATA FLOWERING TOP (UNII: RH041UUZ22) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CAPRYLYL GLYCOL (UNII: 00YIU5438U) STAR ANISE FRUIT (UNII: CK15HA8438) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82224-001-01 430 g in 1 CONTAINER; Type 0: Not a Combination Product 10/27/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/27/2021 Labeler - JM Bio-Tech Co., Ltd. (694081890) Registrant - JM Bio-Tech Co., Ltd. (694081890) Establishment Name Address ID/FEI Business Operations MLS 689850283 manufacture(82224-001)