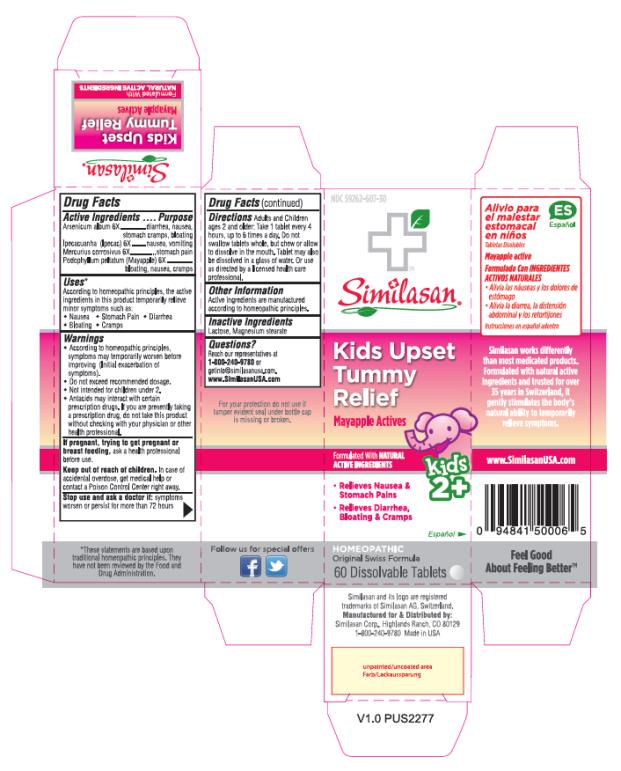
Label: KIDS UPSET TUMMY RELIEF- arsenic trioxide, ipecac, mercuric chloride and podophyllum tablet, orally disintegrating
-
Contains inactivated NDC Code(s)
NDC Code(s): 59262-607-30 - Packager: Similasan Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 12, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Active Ingredients
- Purpose
- Active Ingredients
- Purpose
- Active Ingredients
- Purpose
- Uses*
-
Warnings
• According to homeopathic principles, symptoms may temporarily worsen before improving (Initial exacerbation of symptoms).
• Do not exceed recommended dosage.
• Not intended for children under 2.
• Antacids may interact with certain prescription drugs. If you are presently taking a prescription drug, do not take this product without checking with your physician or other health professional.
- Directions Adults and Children ages 2 and older:
- Other Information
- Inactive Ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KIDS UPSET TUMMY RELIEF
arsenic trioxide, ipecac, mercuric chloride and podophyllum tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59262-607 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 6 [hp_X] IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 6 [hp_X] MERCURIC CHLORIDE (UNII: 53GH7MZT1R) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC CHLORIDE 6 [hp_X] PODOPHYLLUM (UNII: 2S713A4VP3) (PODOPHYLLUM - UNII:2S713A4VP3) PODOPHYLLUM 6 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score no score Shape ROUND Size 8mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59262-607-30 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 02/01/2017 Labeler - Similasan Corporation (111566530)