Label: BALVERSA- erdafitinib tablet, film coated
-
NDC Code(s):
59676-030-56,
59676-030-84,
59676-040-28,
59676-040-56, view more59676-050-28
- Packager: Janssen Products LP
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated February 1, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BALVERSA safely and effectively. See full prescribing information for BALVERSA.
BALVERSA ®(erdafitinib) tablets, for oral use
Initial U.S. Approval: 2019RECENT MAJOR CHANGES
INDICATIONS AND USAGE
BALVERSA is a kinase inhibitor indicated for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma (mUC) with susceptible FGFR3genetic alterations whose disease has progressed on or after at least one line of prior systemic therapy.
Select patients for therapy based on an FDA-approved companion diagnostic for BALVERSA. ( 1, 2.1)
Limitations of Use
BALVERSA is not recommended for the treatment of patients who are eligible for and have not received prior PD-1 or PD-L1 inhibitor therapy. ( 1, 14.1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 3 mg, 4 mg, and 5 mg. ( 3)
CONTRAINDICATIONS
None. ( 4)
WARNINGS AND PRECAUTIONS
- Ocular disorders: BALVERSA can cause central serous retinopathy/retinal pigment epithelial detachment (CSR/RPED). Perform monthly ophthalmological examinations during the first four months of treatment, every 3 months afterwards, and at any time for visual symptoms. Withhold BALVERSA when CSR/RPED occurs and permanently discontinue if it does not resolve within 4 weeks or if Grade 4 in severity. ( 2.3, 5.1)
- Hyperphosphatemia: Increases in phosphate levels are a pharmacodynamic effect of BALVERSA. Monitor for hyperphosphatemia and manage with dose modifications when required. ( 2.3, 5.2)
- Embryo-fetal toxicity: Can cause fetal harm. Advise patients of the potential risk to the fetus and to use effective contraception ( 5.3, 8.1, 8.3)
ADVERSE REACTIONS
The most common (≥20%) adverse reactions, including laboratory abnormalities, were increased phosphate, nail disorders, stomatitis, diarrhea, increased creatinine, increased alkaline phosphatase, increased alanine aminotransferase, decreased hemoglobin, decreased sodium, increased aspartate aminotransferase, fatigue, dry mouth, dry skin, decreased phosphate, decreased appetite, dysgeusia, constipation, increased calcium, dry eye, palmar-plantar erythrodysesthesia syndrome, increased potassium, alopecia, and central serous retinopathy. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Products, LP. at 1-800-526-7736 (1-800-JANSSEN and www.BALVERSA.com) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Moderate CYP2C9 or strong CYP3A4 inhibitors: Consider alternative agents or monitor closely for adverse reactions. ( 7.1)
- Strong CYP3A4 inducers: Avoid concomitant use with BALVERSA. ( 7.1)
- Moderate CYP3A4 inducers: Administer BALVERSA at a dose of 9 mg. ( 7.1)
- Serum phosphate level-altering agents: Avoid concomitant use with agents that can alter serum phosphate levels before the initial dose modification period. ( 2.3, 7.1)
- P-gp substrates: Separate BALVERSA administration by at least 6 hours before or after administration of P-gp substrates with narrow therapeutic indices. ( 7.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Dosage and Schedule
2.3 Dose Modifications for Adverse Reactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Ocular Disorders
5.2 Hyperphosphatemia and Soft Tissue Mineralization
5.3 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on BALVERSA
7.2 Effect of BALVERSA on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 CYP2C9 Poor Metabolizers
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
14 CLINICAL STUDIES
14.1 Urothelial Carcinoma with Susceptible FGFR3Genetic Alterations
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
BALVERSA is indicated for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma (mUC) with susceptible FGFR3genetic alterations whose disease has progressed on or after at least one line of prior systemic therapy.
Select patients for therapy based on an FDA-approved companion diagnostic for BALVERSA [see Dosage and Administration (2.1)and Clinical Studies (14.1)] .
Limitations of Use
BALVERSA is not recommended for the treatment of patients who are eligible for and have not received prior PD-1 or PD-L1 inhibitor therapy [see Clinical Studies (14.1)] .
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients for the treatment of locally advanced or metastatic urothelial carcinoma with BALVERSA based on the presence of susceptible FGFR3genetic alterations in tumor specimens as detected by an FDA-approved companion diagnostic [see Clinical Studies (14.1)] .
Information on FDA-approved tests for the detection of FGFR3genetic alterations in urothelial cancer is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage and Schedule
The recommended starting dose of BALVERSA is 8 mg (two 4 mg tablets) orally once daily, with a dose increase to 9 mg (three 3 mg tablets) once daily based on tolerability, including hyperphosphatemia, at 14 to 21 days [see Dosage and Administration (2.3)].
Swallow tablets whole with or without food. If vomiting occurs any time after taking BALVERSA, the next dose should be taken the next day. Treatment should continue until disease progression or unacceptable toxicity occurs.
If a dose of BALVERSA is missed, it can be taken as soon as possible on the same day. Resume the regular daily dose schedule for BALVERSA the next day. Extra tablets should not be taken to make up for the missed dose.
Dose Increase based on Serum Phosphate Levels
Assess serum phosphate levels 14 to 21 days after initiating treatment. Increase the dose of BALVERSA to 9 mg once daily if serum phosphate level is < 9.0 mg/dL and there are no ocular disorders or Grade 2 or greater adverse reactions. Monitor phosphate levels monthly for hyperphosphatemia [see Pharmacodynamics (12.2)] .
2.3 Dose Modifications for Adverse Reactions
The recommended dose modifications for adverse reactions are listed in Table 1.
Table 1: BALVERSA Dose Reduction Schedule Dose 1 stdose reduction 2 nddose reduction 3 rddose reduction 4 thdose reduction 5 thdose reduction 9 mg ➞
(three 3 mg tablets)8 mg
(two 4 mg tablets)6 mg
(two 3 mg tablets)5 mg
(one 5 mg tablet)4 mg
(one 4 mg tablet)Stop 8 mg ➞
(two 4 mg tablets)6 mg
(two 3 mg tablets)5 mg
(one 5 mg tablet)4 mg
(one 4 mg tablet)Stop Table 2 summarizes recommendations for dose interruption, reduction, or discontinuation of BALVERSA in the management of specific adverse reactions.
Table 2: Dose Modifications for Adverse Reactions Adverse Reaction BALVERSA Dose Modification - *
- Dose adjustment graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAEv5.0).
Hyperphosphatemia In all patients, restrict phosphate intake to 600–800 mg daily. <6.99 mg/dL Continue BALVERSA at current dose. 7–8.99 mg/dL - Continue BALVERSA at current dose.
- Start phosphate binder with food until phosphate level is <7 mg/dL.
- Reduce the dose if serum phosphate remains ≥7 mg/dL for a period of 2 months or if clinically necessary.
9–10 mg/dL - Withhold BALVERSA with weekly reassessments until level returns to <7 mg/dL. Then restart BALVERSA at the same dose level.
- Start phosphate binder with food until serum phosphate level returns to <7 mg/dL.
- Reduce the dose for serum phosphate ≥9 mg/dL for a period of 1 month or if clinically necessary.
>10 mg/dL - Withhold BALVERSA with weekly reassessments until level returns to <7 mg/dL. Then may restart BALVERSA at the first reduced dose level.
- If hyperphosphatemia (≥10 mg/dL) for >2 weeks, discontinue BALVERSA permanently.
- Medical management of symptoms as clinically relevant.
Serum phosphate with life-threatening consequences; urgent intervention indicated (e.g., dialysis) - Discontinue BALVERSA permanently.
Central Serous Retinopathy (CSR) Any Withhold BALVERSA and perform an ophthalmic evaluation within 2 weeks: - If improving within 14 days, restart BALVERSA at the current dose.
- If not improving within 14 days, withhold BALVERSA until improving; once improving, may resume at the next lower dose level.
If recurs or has not improved after 4 weeks of withholding BALVERSA, consider permanent discontinuation.Other Adverse Reactions* Grade 3 Withhold BALVERSA until resolves to Grade 1 or baseline, then may resume dose level lower. Grade 4 Permanently discontinue. -
3 DOSAGE FORMS AND STRENGTHS
Tablets:
- 3 mg: Yellow, round biconvex, film-coated, debossed with "3" on one side; and "EF" on the other side.
- 4 mg: Orange, round biconvex, film-coated, debossed with "4" on one side; and "EF" on the other side.
- 5 mg: Brown, round biconvex, film-coated, debossed with "5" on one side; and "EF" on the other side.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Ocular Disorders
BALVERSA can cause ocular disorders, including central serous retinopathy/retinal pigment epithelial detachment (CSR/RPED) resulting in visual field defect.
In the pooled safety population [see Adverse Reactions (6)] , CSR/RPED occurred in 22% of patients treated with BALVERSA, with a median time to first onset of 46 days. In 104 patients with CSR, 40% required dose interruptions and 56% required dose reductions; 2.9% of BALVERSA-treated patients required permanent discontinuation for CSR. Of the 24 patients who restarted BALVERSA after dose interruption with or without dose reduction, 67% had recurrence and/or worsening of CSR after restarting. CSR was ongoing in 41% of the 104 patients at the time of last evaluation.
Dry eye symptoms occurred in 26% of BALVERSA-treated patients. All patients should receive dry eye prophylaxis with ocular demulcents as needed.
Perform monthly ophthalmological examinations during the first 4 months of treatment and every 3 months afterwards, and urgently at any time for visual symptoms. Ophthalmological examination should include assessment of visual acuity, slit lamp examination, fundoscopy, and optical coherence tomography.
Withhold or permanently discontinue BALVERSA based on severity and/or ophthalmology exam findings [see Dosage and Administration (2.3)] .
5.2 Hyperphosphatemia and Soft Tissue Mineralization
BALVERSA can cause hyperphosphatemia leading to soft tissue mineralization, cutaneous calcinosis, non-uremic calciphylaxis and vascular calcification. Increases in phosphate levels are a pharmacodynamic effect of BALVERSA [see Pharmacodynamics (12.2)].
In the pooled safety population [see Adverse Reactions (6)], increased phosphate occurred in 73% of BALVERSA-treated patients. The median onset time of increased phosphate was 16 days (range: 8–421) after initiating BALVERSA. Twenty-four percent of patients received phosphate binders during treatment with BALVERSA. Vascular calcification was observed in 0.2% of patients treated with BALVERSA.
Monitor for hyperphosphatemia throughout treatment. Restrict dietary phosphate intake (600–800 mg daily) and avoid concomitant use of agents that may increase serum phosphate levels.
If serum phosphate is above 7.0 mg/dL, consider adding an oral phosphate binder until serum phosphate level returns to <7.0 mg/dL. Withhold, dose reduce, or permanently discontinue BALVERSA based on duration and severity of hyperphosphatemia according to Table 2 [see Dosage and Administration (2.3)].
5.3 Embryo-Fetal Toxicity
Based on the mechanism of action and findings in animal reproduction studies, BALVERSA can cause fetal harm when administered to a pregnant woman. In an embryo-fetal toxicity study, oral administration of erdafitinib to pregnant rats during the period of organogenesis caused malformations and embryo-fetal death at maternal exposures that were less than the human exposures at the maximum human recommended dose based on area under the curve (AUC). Advise pregnant women of the potential risk to the fetus. Advise female patients of reproductive potential to use effective contraception during treatment with BALVERSA and for one month after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with BALVERSA and for one month after the last dose [see Use in Specific Populations (8.1, 8.3)and Clinical Pharmacology (12.1)] .
-
6 ADVERSE REACTIONS
The following serious adverse reactions are also described elsewhere in the labeling:
- Ocular Disorders [see Warnings and Precautions (5.1)] .
- Hyperphosphatemia [see Warnings and Precautions (5.2)] .
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in the WARNINGS AND PRECAUTIONS reflects exposure to BALVERSA as a single agent at the recommended dose (8 to 9 mg orally daily) in 479 patients with advanced urothelial cancer and FGFRalterations in 42756493BLC3001 (NCT03390504), 42756493BLC2001 (NCT02365597), 42756493BLC2002 (NCT 03473743), and 42756493EDI1001 (NCT01703481). Among 479 patients who received BALVERSA, the median duration of treatment was 4.8 months (range: 0.1 to 43 months). In this pooled safety population, the most common (>20%) adverse reactions, including laboratory abnormalities, were increased phosphate, nail disorders, stomatitis, diarrhea, increased creatinine, increased alkaline phosphatase, increased alanine aminotransferase, decreased hemoglobin, decreased sodium, increased aspartate aminotransferase, fatigue, dry mouth, dry skin, decreased phosphate, decreased appetite, dysgeusia, constipation, increased calcium, dry eye, palmar-plantar erythrodysesthesia syndrome, increased potassium, alopecia, and central serous retinopathy.
BLC3001
The safety of BALVERSA was evaluated in Cohort 1 of the BLC3001 study that included patients with locally advanced unresectable or metastatic urothelial carcinoma which had susceptible FGFR3genetic alterations and were previously treated with a PD-1 or PD-L1 inhibitor [see Clinical Studies (14.1)] . Patients received either BALVERSA (8 mg orally once daily with individualized up-titration to 9 mg) (n=135) or chemotherapy (docetaxel 75 mg/m 2once every 3 weeks or vinflunine 320 mg/m 2once every 3 weeks) (n=112). Among patients who received BALVERSA, median duration of treatment was 4.8 months (range: 0.2 to 38 months).
Serious adverse reactions occurred in 41% of patients who received BALVERSA. Serious reactions in >2% of patients included urinary tract infection (4.4%), hematuria (3.7%), hyponatremia (2.2%), and acute kidney injury (2.2%). Fatal adverse reactions occurred in 4.4% of patients who received BALVERSA, including sudden death (1.5%), pneumonia (1.5%), renal failure (0.7%), and cardiorespiratory arrest (0.7%).
Permanent discontinuation of BALVERSA due to an adverse reaction occurred in 14% of patients. Adverse reactions which resulted in permanent discontinuation of BALVERSA in >2% of patients included nail disorders (3%) and eye disorders (2.2%).
Dosage interruptions of BALVERSA due to an adverse reaction occurred in 72% of patients. Adverse reactions which required dosage interruption in >4% of patients included nail disorders (22%), stomatitis (19%), eye disorders (16%), palmar-plantar erythrodysesthesia syndrome (15%), diarrhea (10%), hyperphosphatemia (7%), increased aspartate aminotransferase (6%), and increased alanine aminotransferase (5%).
Dose reductions of BALVERSA due to an adverse reaction occurred in 69% of patients. Adverse reactions which required dose reductions in >4% of patients included nail disorders (27%), stomatitis (19%), eye disorders (17%), palmar-plantar erythrodysesthesia syndrome (12%), diarrhea (7%), dry mouth (4.4%), and hyperphosphatemia (4.4%).
Table 3 presents adverse reactions reported in ≥15% of patients treated with BALVERSA at 8 or 9 mg once daily versus chemotherapy.
Table 3: Adverse Reactions Reported in ≥15% of Patients Who Received BALVERSA Versus Chemotherapy (Study BLC3001) Adverse Reaction BALVERSA (N=135) Chemotherapy (N=112) All Grades (%) Grade 3–4 (%) All Grades (%) Grade 3–4 (%) - *
- Includes multiple terms
Skin and subcutaneous tissue disorders Nail disorders * 70 12 5 0 Palmar-plantar erythrodysesthesia syndrome 30 10 0.9 0 Dry skin * 27 1.5 6 0 Alopecia 25 0.7 24 0 Gastrointestinal disorders Diarrhea * 63 3 17 2.7 Stomatitis * 56 10 18 1.8 Dry Mouth 39 0 3.6 0 Constipation 27 0 28 1.8 Nervous system disorders Dysgeusia * 30 0.7 7 0 General disorders Fatigue * 29 1.5 42 7 Metabolism and nutrition disorders Decreased appetite 27 3 21 2.7 Eye disorders Dry eye * 25 0.7 3.6 0 Central serous retinopathy * 18 2.2 0 0 Investigations Decreased weight 22 2 2.7 0 Clinically relevant adverse reactions in <15% of patients who received BALVERSA included nausea (15%), pyrexia (15%), epistaxis (13%), vomiting (10%), and arthralgia (10%).
Table 4 presents laboratory abnormalities reported in ≥15% of patients treated with BALVERSA at 8 or 9 mg once daily versus chemotherapy.
Table 4: Selected Laboratory Abnormalities Reported in ≥15% of Patients Who Received BALVERSA Versus Chemotherapy; Cohort 1 Safety Analysis Set (Study BLC3001) Laboratory Abnormality BALVERSA (N=135 *) Chemotherapy (N=112 †) All Grades ‡(%) Grade 3–4 ‡(%) All Grades ‡(%) Grade 3–4 ‡(%) - *
- The denominator used to calculate the rate varied from 52 to 131 based on the number of patients with a baseline value and at least one post-treatment value.
- †
- The denominator used to calculate the rate varied from 11 to 102 based on the number of patients with a baseline value and at least one post-treatment value.
- ‡
- Severity graded per NCI CTCAE v4.03.
Chemistry Increased phosphate 76 5 0 0 Increased alkaline phosphatase 54 4.7 29 1 Increased alanine aminotransferase 46 3.8 15 1 Increased aspartate aminotransferase 44 3.1 13 0 Decreased sodium 44 16 25 6 Increased creatinine 43 1.5 17 0 Decreased phosphate 34 8 25 3.6 Increased calcium 27 8 9 0 Increased potassium 24 0 21 0 Hematology Decreased hemoglobin 50 12 57 12 Decreased platelet count 17 1.5 18 1 Decreased neutrophil count 16 0.8 40 26 BLC2001
The safety of BALVERSA was evaluated in the BLC2001 study that included 87 patients with locally advanced or metastatic urothelial carcinoma which had susceptible FGFR3and other FGFRalterations, and which progressed during or following at least one line of prior chemotherapy including within 12 months of neoadjuvant or adjuvant chemotherapy [see Clinical Studies (14.1)] . Patients were treated with BALVERSA at 8 mg orally once daily; with a dose increase to 9 mg in patients with phosphate levels <5.5 mg/dL on Day 14 of Cycle 1. Median duration of treatment was 5.3 months (range: 0 to 17 months).
Serious adverse reactions occurred in 41% of patients. The most frequent (>3%) serious adverse reactions were central serous retinopathy (4.6%), urinary tract infection (3.4%), and general physical health deterioration (3.4%).
Fatal adverse reactions occurred in 8% of patients, including acute myocardial infarction (1.1%).
Permanent discontinuation of BALVERSA due to an adverse reaction occurred in 21% of patients. The most frequent (≥ 2%) reasons for permanent discontinuation included central serous retinopathy (4.6%), general physical health deterioration (3.4%), palmar-plantar erythrodysesthesia syndrome (2.3%), acute kidney injury (2.3%), and fatigue (2.3%).
Dosage interruptions of BALVERSA occurred in 68% of patients. The most frequent (≥ 5%) adverse reactions requiring dosage interruption included hyperphosphatemia (24%), stomatitis (17%), nail disorders (16%), central serous retinopathy (9%), palmar-plantar erythro-dysesthesia syndrome (8%), and fatigue (8%).
Dose reductions of BALVERSA occurred in 53% of patients. The most frequent (≥ 5%) adverse reactions for dose reductions included nail disorders (21%), stomatitis (15%), central serous retinopathy (14%), hyperphosphatemia (7%), palmar-plantar erythro-dysesthesia syndrome (7%), fatigue (6%), and blurred vision (6%).
Table 5 presents adverse reactions reported in ≥15% of patients treated with BALVERSA at 8 mg or 9 mg once daily.
Table 5: Adverse Reactions Reported in ≥15% of Patients (Study BLC2001) Adverse Reaction BALVERSA 8 mg daily (N=87) All Grades (%) Grade 3–4 (%) Gastrointestinal disorders Stomatitis * 62 11 Diarrhea * 48 4.6 Dry mouth 45 0 Constipation 28 1.1 Nausea 21 1.1 Skin and subcutaneous tissue disorders Nail disorders * 62 14 Dry skin * 37 0 Alopecia 26 0 Palmar-plantar erythrodysesthesia syndrome 26 6 General disorders and admin. site conditions Fatigue *,† 54 8 Decreased weight 16 0 Metabolism and nutrition disorders Decreased appetite 38 0.0 Nervous system disorders Dysgeusia * 38 1.1 Eye disorders Dry eye * 29 1.1 Central serous retinopathy * 28 4.6 Blurred vision 17 0 Infections and Infestations Urinary tract infection 17 6 Clinically relevant adverse reactions in <15% of patients who received BALVERSA included pyrexia (14%), extremity pain (13%), vomiting (13%), and peripheral edema (10%).
Table 6 presents laboratory abnormalities reported in ≥15% of patients treated with BALVERSA at 8 mg or 9 mg once daily.
Table 6: Selected Laboratory Abnormalities Reported in ≥ 15% of Patients Laboratory Abnormality BALVERSA 8 mg daily (N=87 *) All Grades (%) Grade 3–4 (%) - *
- The denominator used to calculate the rate varied from 83 to 86 based on the number of patients with a baseline value and at least one post-treatment value.
Chemistry Increased phosphate 76 1.2 Increased creatinine 52 4.7 Increased alanine aminotransferase 41 1.2 Increased alkaline phosphatase 41 1.2 Decreased sodium 40 16 Decreased magnesium 31 1.2 Increased aspartate aminotransferase 30 0 Decreased phosphate 24 9 Increased calcium 22 3.5 Hematology Decreased hemoglobin 35 3.5 Decreased platelets 19 1.2 Decreased leukocytes 17 0 -
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on BALVERSA
Table 7 summarizes drug interactions that affect the exposure of BALVERSA or serum phosphate level and their clinical management.
Table 7: Drug Interactions that Affect BALVERSA Moderate CYP2C9 or Strong CYP3A4 Inhibitors Clinical Impact - Co-administration of BALVERSA with moderate CYP2C9 or strong CYP3A4 inhibitors increased erdafitinib plasma concentrations [see Clinical Pharmacology (12.3)].
- Increased erdafitinib plasma concentrations may lead to increased drug-related toxicity [see Warnings and Precautions (5)].
Clinical Management - Consider alternative therapies that are not moderate CYP2C9 or strong CYP3A4 inhibitors during treatment with BALVERSA.
- If co-administration of a moderate CYP2C9 or strong CYP3A4 inhibitor is unavoidable, monitor closely for adverse reactions and consider dose modifications accordingly [see Dosage and Administration (2.3)]. If the moderate CYP2C9 or strong CYP3A4 inhibitor is discontinued, resume the BALVERSA dose before dose modifications in the absence of drug-related toxicity.
Strong CYP3A4 Inducers Clinical Impact - Co-administration of BALVERSA with strong CYP3A4 inducers decreased erdafitinib plasma concentrations [see Clinical Pharmacology (12.3)].
- Decreased erdafitinib plasma concentrations may lead to decreased activity.
Clinical Management - Avoid co-administration of strong CYP3A4 inducers with BALVERSA.
Moderate CYP3A4 Inducers Clinical Impact - Co-administration of BALVERSA with moderate CYP3A4 inducers may decrease erdafitinib plasma concentrations [see Clinical Pharmacology (12.3)].
- Decreased erdafitinib plasma concentrations may lead to decreased activity.
Clinical Management - If a moderate CYP3A4 inducer must be co-administered at the start of BALVERSA treatment, administer BALVERSA at a dose of 9 mg daily.
- When a moderate CYP3A4 inducer is discontinued, continue BALVERSA at the same dose, in the absence of drug-related toxicity.
Serum Phosphate Level-Altering Agents Clinical Impact - Co-administration of BALVERSA with other serum phosphate level-altering agents may increase or decrease serum phosphate levels [see Pharmacodynamics (12.2)].
- Changes in serum phosphate levels due to serum phosphate level-altering agents (other than erdafitinib) may interfere with serum phosphate levels needed for the determination of initial dose increased based on serum phosphate levels [see Dosage and Administration (2.3)].
Clinical Management - Avoid co-administration of serum phosphate level-altering agents with BALVERSA before initial dose increase period based on serum phosphate levels (Days 14 to 21) [see Dosage and Administration (2.3)].
7.2 Effect of BALVERSA on Other Drugs
Table 8 summarizes the effect of BALVERSA on other drugs and their clinical management.
Table 8: BALVERSA Drug Interactions that Affect Other Drugs P-glycoprotein (P-gp) Substrates Clinical Impact - Co-administration of BALVERSA with P-gp substrates may increase the plasma concentrations of P-gp substrates [see Clinical Pharmacology (12.3)].
- Increased plasma concentrations of P-gp substrates may lead to increased toxicity of the P-gp substrates.
Clinical Management - If co-administration of BALVERSA with P-gp substrates is unavoidable, separate BALVERSA administration by at least 6 hours before or after administration of P-gp substrates with narrow therapeutic index.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on the mechanism of action and findings in animal reproduction studies, BALVERSA can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)] . There are no available data on BALVERSA use in pregnant women to inform a drug-associated risk. Oral administration of erdafitinib to pregnant rats during organogenesis caused malformations and embryo-fetal death at maternal exposures that were less than the human exposures at the maximum recommended human dose based on AUC (see Data). Advise pregnant women and females of reproductive potential of the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Data
Animal Data
In an embryo-fetal toxicity study, erdafitinib was orally administered to pregnant rats during the period of organogenesis. Doses ≥4 mg/kg/day (at total maternal exposures <0.1% of total human exposures at the maximum recommended human dose based on AUC) produced embryo-fetal death, major blood vessel malformations and other vascular anomalies, limb malformations (ectrodactyly, absent or misshapen long bones), an increased incidence of skeletal anomalies in multiple bones (vertebrae, sternebrae, ribs), and decreased fetal weight.
8.2 Lactation
Risk Summary
There are no data on the presence of erdafitinib in human milk, or the effects of erdafitinib on the breastfed child, or on milk production. Because of the potential for serious adverse reactions from erdafitinib in a breastfed child, advise lactating women not to breastfeed during treatment with BALVERSA and for one month following the last dose.
8.3 Females and Males of Reproductive Potential
BALVERSA can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)] .
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating treatment with BALVERSA.
Contraception
Infertility
Females
Based on findings from animal studies, BALVERSA may impair fertility in females of reproductive potential [see Nonclinical Toxicology (13.1)] .
8.4 Pediatric Use
Safety and effectiveness of BALVERSA in pediatric patients have not been established.
In 4 and 13-week repeat-dose toxicology studies in rats and dogs, toxicities in bone and teeth were observed at an exposure less than the human exposure (AUC) at the maximum recommended human dose. Chondroid dysplasia/metaplasia were reported in multiple bones in both species, and tooth abnormalities included abnormal/irregular denting in rats and dogs and discoloration and degeneration of odontoblasts in rats.
8.5 Geriatric Use
Of the 479 patients treated with BALVERSA in clinical studies, 40% of patients were less than 65 years old, 40% of patients were 65 years to 74 years old, and 20% were 75 years old and over.
Patients 65 years of age and older treated with BALVERSA experienced a higher incidence of adverse reactions requiring treatment discontinuation than younger patients. In clinical trials, the incidence of treatment discontinuations of BALVERSA due to adverse reactions was 10% in patients younger than 65 years, 20% in patients ages 65–74 years, and 35% in patients 75 years or older.
No overall difference in efficacy was observed between these patients and younger patients [see Clinical Studies (14.1)].
8.6 CYP2C9 Poor Metabolizers
CYP2C9*3/*3 Genotype:Erdafitinib plasma concentrations are predicted to be higher in patients with the CYP2C9*3/*3 genotype. Monitor for increased adverse reactions in patients who are known or suspected to have CYP2C9*3/*3 genotype [see Pharmacogenomics (12.5)] .
-
11 DESCRIPTION
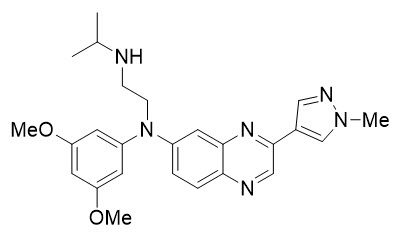
Erdafitinib, the active ingredient in BALVERSA, is a kinase inhibitor. The chemical name is N-(3,5-dimethoxyphenyl)-N'-(1-methylethyl)-N-[3-(1-methyl-1H-pyrazol-4-yl)quinoxalin-6-yl]ethane-1,2-diamine. Erdafitinib is a yellow powder. It is practically insoluble, or insoluble to freely soluble in organic solvents, and slightly soluble to practically insoluble, or insoluble in aqueous media over a wide range of pH values. The molecular formula is C 25H 30N 6O 2and molecular weight is 446.56.
Chemical structure of erdafitinib is as follows:
BALVERSA ®(erdafitinib) tablets are supplied as 3 mg, 4 mg or 5 mg film-coated tablets for oral administration and contains the following inactive ingredients:
Tablet Core: Croscarmellose sodium, Magnesium stearate (from vegetable source), Mannitol, Meglumine, and Microcrystalline Cellulose.
Film Coating: (Opadry amb II): Glycerol monocaprylocaprate Type I, Polyvinyl alcohol-partially hydrolyzed, Sodium lauryl sulfate, Talc, Titanium dioxide, Iron oxide yellow, Iron oxide red (for the orange and brown tablets only), Ferrosoferric oxide/iron oxide black (for the brown tablets only).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Erdafitinib is a kinase inhibitor that binds to and inhibits enzymatic activity of FGFR1, FGFR2, FGFR3 and FGFR4 based on in vitrodata. Erdafitinib inhibited FGFR phosphorylation and signaling and decreased cell viability in cell lines expressing FGFRgenetic alterations, including point mutations, amplifications, and fusions. Erdafitinib demonstrated antitumor activity in FGFR-expressing cell lines and xenograft models derived from tumor types, including bladder cancer.
12.2 Pharmacodynamics
Cardiac Electrophysiology
Based on evaluation of QTc interval in an open-label, dose escalation and dose expansion study in 187 patients with cancer, erdafitinib had no large effect (i.e., > 20 ms) on the QTc interval.
Serum Phosphate
FGFR inhibition by BALVERSA increases serum phosphate level [see Dosage and Administration (2.3)and Drug Interactions (7.1)].
12.3 Pharmacokinetics
Following administration of BALVERSA 8 mg once daily, the mean (coefficient of variation [CV%]) erdafitinib steady-state maximum plasma concentration (C max), area under the curve (AUC tau), and minimum plasma concentration (C min) were 1,399 ng/mL (51%), 29,268 ng∙h/mL (60%), and 936 ng/mL (65%), respectively.
Following single and repeat once daily dosing of BALVERSA, erdafitinib exposure (C maxand AUC) increased proportionally across the dose range of 0.5 to 12 mg (0.06 to 1.3 times the maximum approved recommended dose). Steady state was achieved after 2 weeks with once daily dosing with a mean accumulation ratio was 4-fold.
Absorption
Median time to achieve peak plasma concentration (t max) was 2.5 hours (range: 2 to 6 hours).
Distribution
The mean apparent volume of distribution of erdafitinib was 29 L.
Erdafitinib protein binding was 99.7% in patients, primarily to alpha-1-acid glycoprotein.
Elimination
The mean total apparent clearance (CL/F) of erdafitinib was 0.362 L/h.
The mean effective half-life of erdafitinib was 59 hours.
Metabolism
Erdafitinib is primarily metabolized by CYP2C9 and CYP3A4. The contribution of CYP2C9 and CYP3A4 in the total clearance of erdafitinib is estimated to be 39% and 20%, respectively. Unchanged erdafitinib was the major drug-related moiety in plasma, there were no circulating metabolites.
Excretion
Following a single oral dose of radiolabeled erdafitinib, approximately 69% of the dose was recovered in feces (19% as unchanged) and 19% in urine (13% as unchanged).
Specific Populations
No clinically meaningful effects on erdafitinib exposure were observed based on age (21–92 years), sex, race (White, Hispanic or Asian), body weight (36–166 kg), mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, or mild to moderate renal impairment (eGFR 30 to 89 mL/min/1.73 m²). Limited data are available in patients with severe (Child-Pugh C) hepatic impairment and in patients with severe renal impairment. The pharmacokinetics of erdafitinib in patients with renal impairment requiring dialysis is unknown.
Drug Interaction Studies
Clinical Studies
Effect of Other Drugs on Erdafitinib
Moderate CYP2C9 Inhibitors
Erdafitinib mean ratios for C maxand AUC infwere 121% and 148%, respectively, when BALVERSA was co-administered with fluconazole, a moderate CYP2C9 and CYP3A4 inhibitor, relative to BALVERSA administered alone.
In Vitro Studies
CYP Substrates
Erdafitinib is a time dependent inhibitor and inducer of CYP3A4. Erdafitinib is not an inhibitor of other major CYP isozymes at clinically relevant concentrations.
Transporters
Erdafitinib is a substrate and inhibitor of P-gp. P-gp inhibitors are not expected to affect erdafitinib exposure to a clinically relevant extent. Erdafitinib is an inhibitor of OCT2.
Erdafitinib does not inhibit BCRP, OATP1B, OATP1B3, OAT1, OAT3, OCT1, MATE-1, or MATE-2K at clinically relevant concentrations.
12.5 Pharmacogenomics
CYP2C9 activity is reduced in individuals with genetic variants, such as the CYP2C9*2 and CYP2C9*3 polymorphisms. Erdafitinib exposure was similar in subjects with CYP2C9*1/*2 and *1/*3 genotypes relative to subjects with CYP2C9*1/*1 genotype (wild type). No data are available in subjects characterized by other genotypes (e.g., *2/*2, *2/*3, *3/*3). Simulation suggested no clinically meaningful differences in erdafitinib exposure in subjects with CYP2C9*2/*2 and *2/*3 genotypes. The exposure of erdafitinib is predicted to be 50% higher in subjects with the CYP2C9*3/*3 genotype, estimated to be present in 0.4% to 3% of the population among various ethnic groups.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenicity studies have not been conducted with erdafitinib.
Erdafitinib was not mutagenic in a bacterial reverse mutation (Ames) assay and was not clastogenic in an in vitromicronucleus or an in vivorat bone marrow micronucleus assay.
Fertility studies in animals have not been conducted with erdafitinib. In the 3-month repeat-dose toxicity study, erdafitinib showed effects on female reproductive organs (necrosis of the ovarian corpora lutea) in rats at an exposure less than the human exposure (AUC) at maximum recommended human dose.
-
14 CLINICAL STUDIES
14.1 Urothelial Carcinoma with Susceptible FGFR3Genetic Alterations
The efficacy of BALVERSA was evaluated in Study BLC3001 (NCT03390504) Cohort 1, a randomized, open-label, multicenter study in which 266 patients with advanced urothelial cancer harboring selected FGFR3alterations were randomized 1:1 to receive BALVERSA (8 mg with titration up to 9 mg) versus chemotherapy (docetaxel 75 mg/m 2once every 3 weeks or vinflunine 320 mg/m 2once every 3 weeks) until unacceptable toxicity or progression. Randomization was stratified by region (North America vs. Europe vs. rest of world), Eastern Cooperative Oncology Group (ECOG) performance status (0 or 1 vs. 2) and visceral or bone metastases (yes vs. no). All patients needed to have had disease progression after 1 or 2 prior treatments, at least 1 of which included a PD-1 or PD-L1 inhibitor. FGFR3genetic alterations were identified from tumor tissue in a central laboratory by the QIAGEN therascreen®FGFRRGQ RT-Polymerase Chain Reaction (PCR) kit in 75% of patients while the remainder (25%) were identified by local next generation sequencing (NGS) assays.
The major efficacy outcome measures were overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) assessed by investigator using RECIST (Response Evaluation Criteria in Solid Tumors) Version 1.1.
The median age was 67 years (range: 32 to 86 years) and 71% were male; 54% were White, 29% Asian, 0.4% Black, 0.4% multiple races, 16% not reported; 2% were Hispanic/Latino; and baseline ECOG performance status was 0 (43%), 1 (48%), or 2 (9%). Eighty-one percent of patients had FGFR3mutations, 17% had fusions, and 2% had both mutations and fusions. Ninety-five percent of patients had pure transitional cell carcinoma (TCC) and 5% had TCC with other histologic variants. The primary tumor location was the upper tract for 33% of subjects and lower tract for 67%; 74% of patients had visceral or bone metastases. Eighty-eight percent of patients received platinum-containing chemotherapy previously. PD-1 or PD-L1 inhibitor therapy was received only in the neoadjuvant or adjuvant setting in 7% of patients.
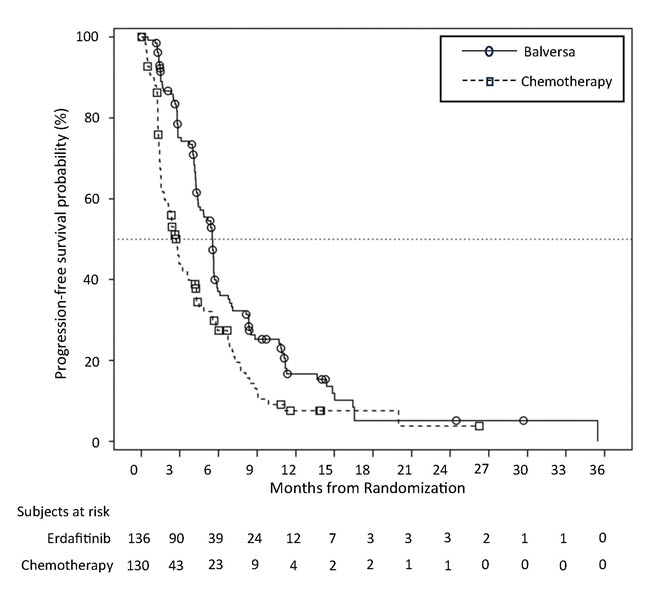
Statistically significant improvements in OS, PFS, and ORR were demonstrated for BALVERSA compared with chemotherapy.
Table 9 and Figures 1 and 2 summarize the efficacy results for BLC3001 Cohort 1.
Table 9: Efficacy Results for Study BLC3001 Cohort 1 BALVERSA
N=136Chemotherapy
N=130All p-values reported are 2-sided and compared with 0.019 of the allocated alpha for the interim analysis.
ORR = confirmed objective response (CR + PR)
CI = Confidence IntervalOverall Survival (OS) Number of events (%) 77 (56.6%) 78 (60.0%) Median *, months (95% CI) 12.1 (10.3, 16.4) 7.8 (6.5, 11.1) Hazard ratio †(95% CI) 0.64 (0.47, 0.88) p-value ‡ 0.0050 Progression-free survival (PFS) Number of events (%) 101 (74.3%) 90 (69.2%) Median *, months (95% CI) 5.6 (4.4, 5.7) 2.7 (1.8, 3.7) Hazard ratio †(95% CI) 0.58 (0.44, 0.78) p-value ‡ 0.0002 Objective response rate (ORR) ORR (95% CI) 35.3% (27.3, 43.9) 8.5% (4.3, 14.6) p-value § <0.001 Complete response, CR (%) 5.1% 0.8% Partial response, PR (%) 30.1% 7.7% Figure 1: Kaplan-Meier Plot of Overall Survival (Study BLC3001 Cohort 1)
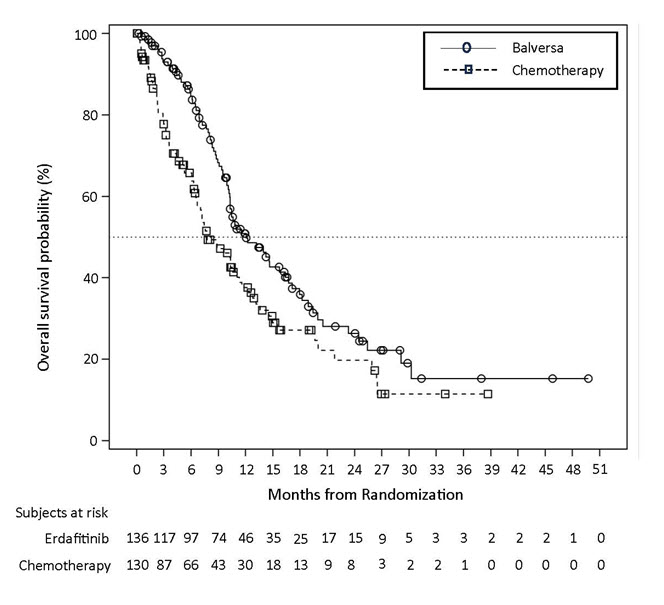
Figure 2: Kaplan-Meier Plot of Progression-free Survival (Study BLC3001 Cohort 1)
Study BLC3001 Cohort 2
Study BLC3001 (NCT03390504) Cohort 2 was a multicenter, open-label, randomized study in 351 patients with locally advanced or metastatic urothelial carcinoma with selected FGFR3alterations who received 1 prior line of systemic therapy and no prior PD-1 or PD-L1 inhibitor. Patients were randomized 1:1 to receive BALVERSA (8 mg with titration up to 9 mg) or pembrolizumab 200 mg every 3 weeks. The study did not meet its major efficacy outcome measure for superiority of OS at the pre-specified final analysis. The OS hazard ratio (HR) was 1.18 (95% CI: 0.92, 1.51; p=0.18), median 10.9 (95% CI: 9.2, 12.6) months for BALVERSA versus 11.1 (95% CI: 9.7, 13.6) months for pembrolizumab [see Indications and Usage (1)] .
Study BLC2001
Study BLC2001 (NCT02365597) was a multicenter, open-label, single-arm study to evaluate the efficacy and safety of BALVERSA in patients with locally advanced or metastatic urothelial carcinoma (mUC). FGFRmutation status for screening and enrollment of patients was determined by a clinical trial assay (CTA). The efficacy population consists of a cohort of eighty-seven patients who were enrolled in this study with disease that had progressed on or after at least one prior chemotherapy and that had at least 1 of the following genetic alterations: FGFR3gene mutations ( R248C, S249C, G370C, Y373C) or FGFRgene fusions ( FGFR3-TACC3, FGFR3-BAIAP2L1, FGFR2-BICC1, FGFR2-CASP7), as determined by the CTA performed at a central laboratory. Tumor samples from 69 patients were tested retrospectively by the QIAGEN therascreen®FGFRRGQ RT-PCR Kit, which is the FDA-approved test for selection of patients with mUC for BALVERSA.
Patients received a starting dose of BALVERSA at 8 mg once daily with a dose increase to 9 mg once daily in patients whose serum phosphate levels were below the target of 5.5 mg/dL between days 14 and 17; a dose increase occurred in 41% of patients. BALVERSA was administered until disease progression or unacceptable toxicity. The major efficacy outcome measures were ORR and duration of response (DoR), as determined by blinded independent review committee (BIRC) according to RECIST v1.1.
The median age was 67 years (range: 36 to 87 years), 79% were male, and 74% were Caucasian. Most patients (92%) had a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Sixty-six percent of patients had visceral metastases. Eighty-four (97%) patients received at least one of cisplatin or carboplatin previously. Fifty-six percent of patients only received prior cisplatin-based regimens, 29% received only prior carboplatin-based regimens, and 10% received both cisplatin and carboplatin-based regimens. Three (3%) patients had disease progression following prior platinum-containing neoadjuvant or adjuvant therapy only. Twenty-four percent of patients had been treated with prior anti PD-L1/PD-1 therapy.
Efficacy results are summarized in Table 10 and Table 11. ORR was 32.2%. Responders included patients who had previously not responded to anti PD-L1/PD-1 therapy.
Table 10: Efficacy Results Endpoint BIRC *Assessment N=87 ORR = CR + PR
CI = Confidence Interval- *
- BIRC: Blinded Independent Review Committee
ORR (95% CI) 32.2% (22.4, 42.0) Complete response (CR) 2.3% Partial response (PR) 29.9% Median DoR in months (95% CI) 5.4 (4.2, 6.9) -
16 HOW SUPPLIED/STORAGE AND HANDLING
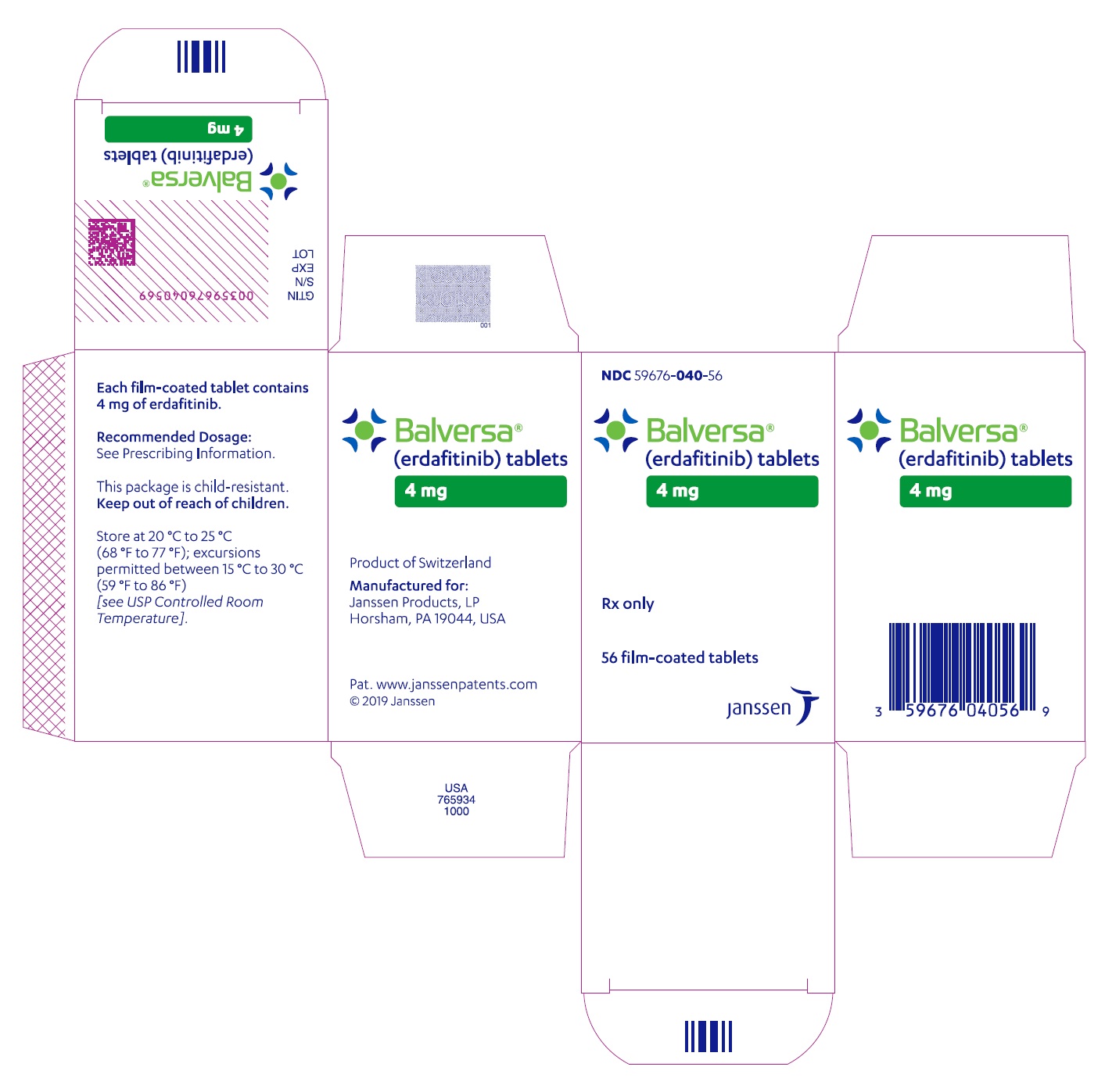
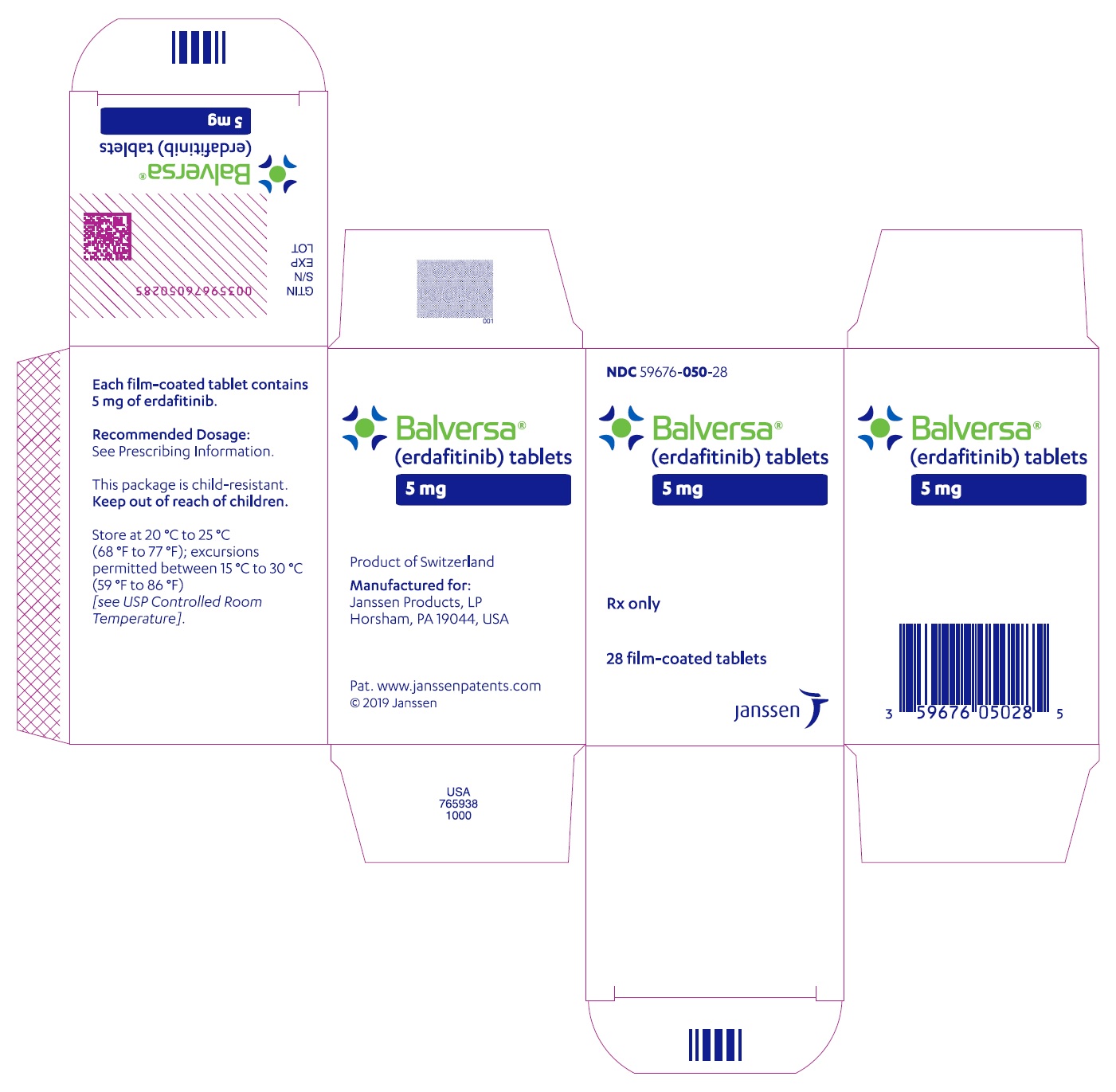
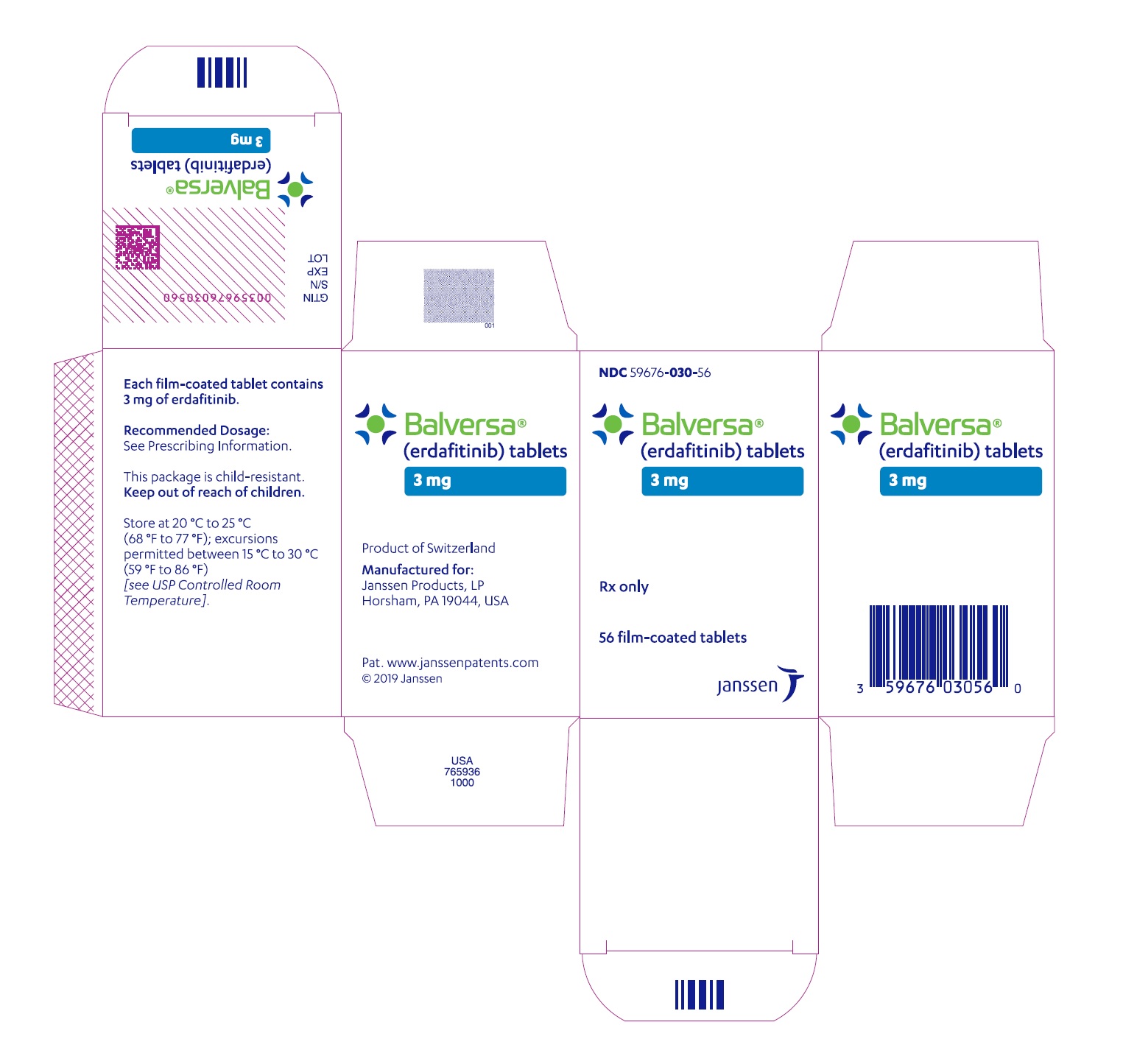
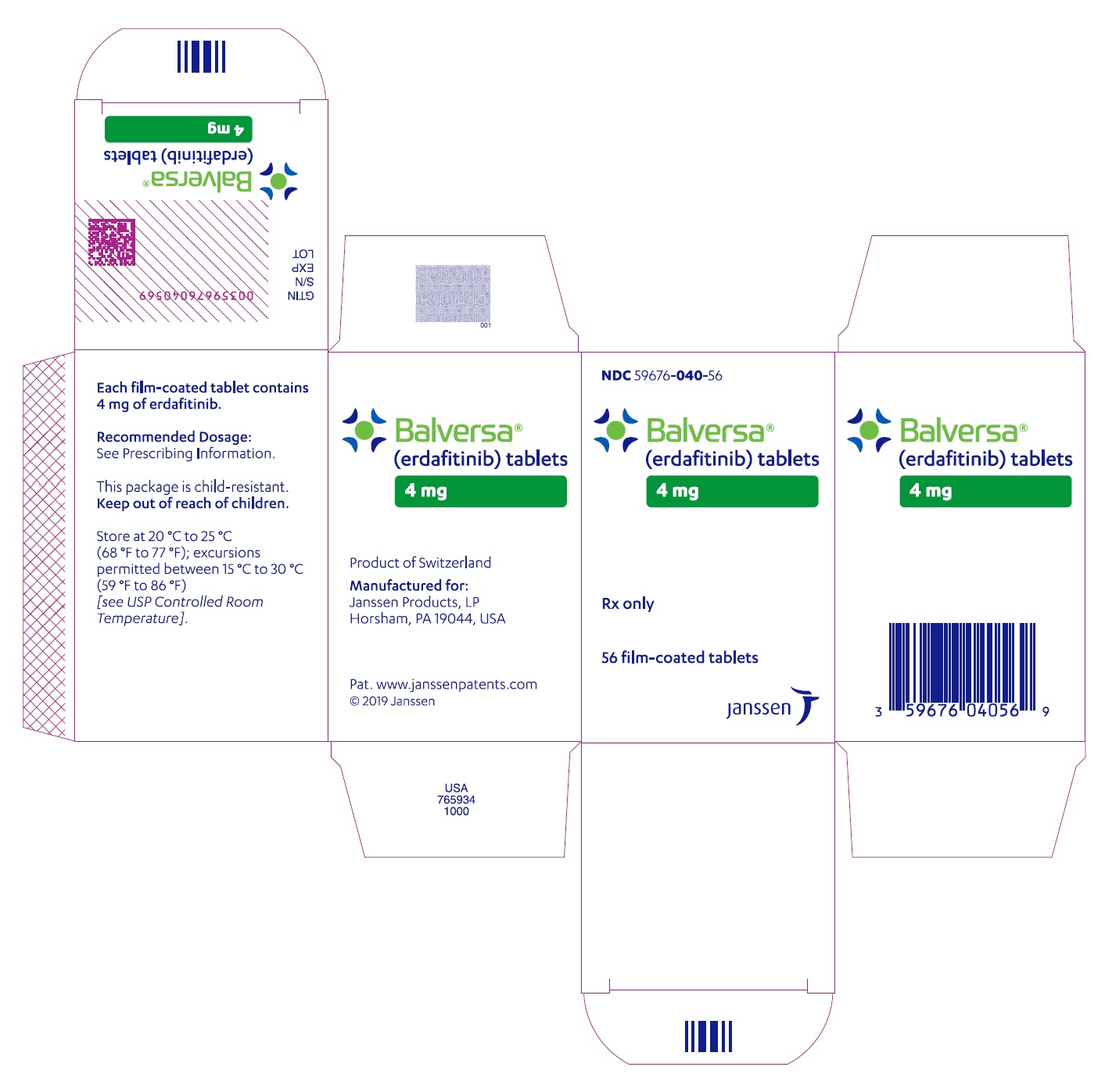
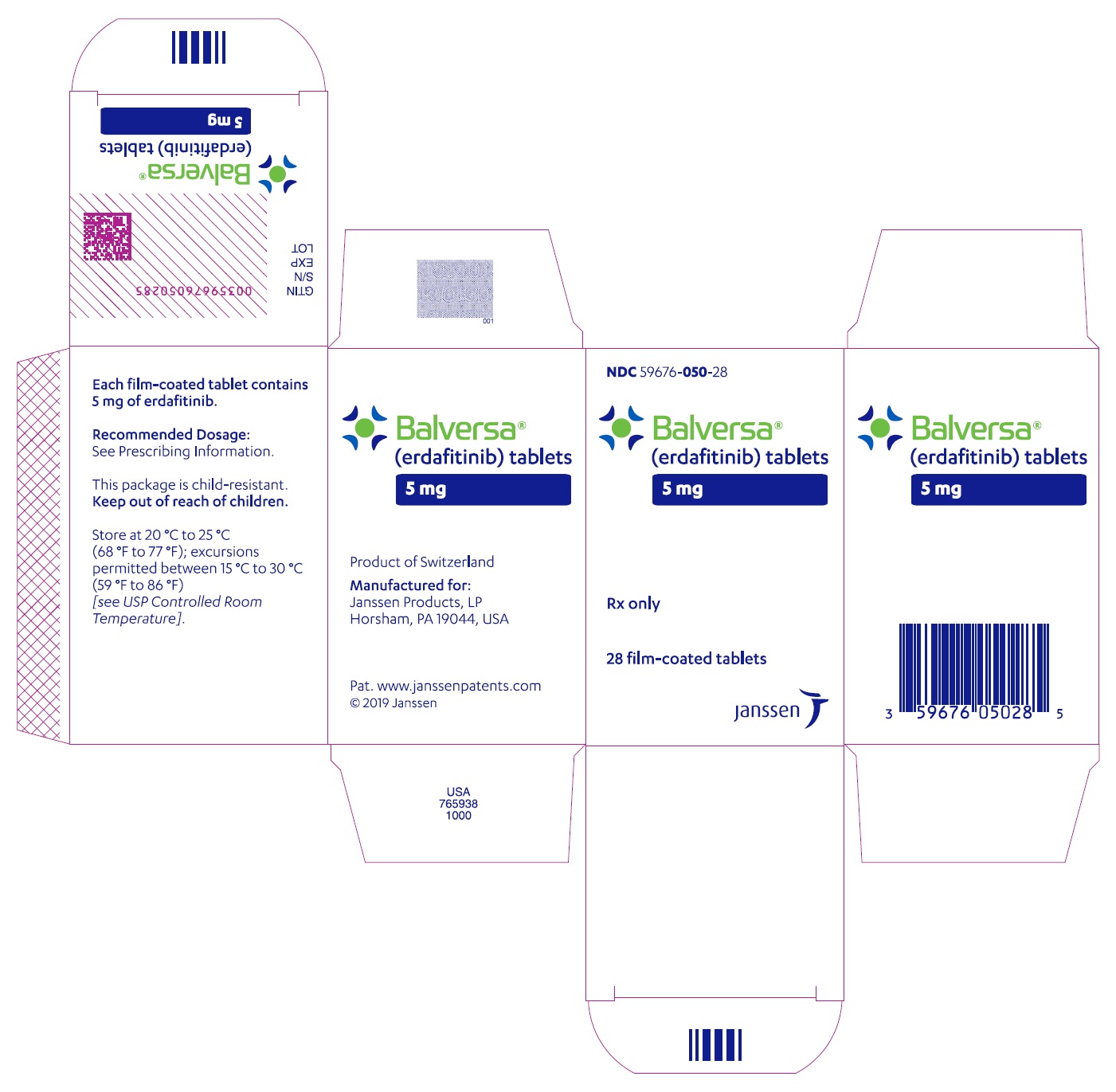
BALVERSA ®(erdafitinib) tablets are available in the strengths and packages listed below:
- 3 mg tablets: Yellow, round biconvex, film-coated, debossed with "3" on one side and "EF" on the other side.
- Bottle of 56-tablets with child resistant closure (NDC 59676-030-56).
- Bottle of 84-tablets with child resistant closure (NDC 59676-030-84).
- 4 mg tablets: Orange, round biconvex, film-coated, debossed with "4" on one side and "EF" on the other side.
- Bottle of 28-tablets with child resistant closure (NDC 59676-040-28).
- Bottle of 56-tablets with child resistant closure (NDC 59676-040-56).
- 5 mg tablets: Brown, round biconvex, film-coated, debossed with "5" on one side and "EF" on the other side.
- Bottle of 28-tablets with child resistant closure (NDC 59676-050-28).
- 3 mg tablets: Yellow, round biconvex, film-coated, debossed with "3" on one side and "EF" on the other side.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
FGFRGenetic Alterations
Advise patients that evidence of a susceptible FGFR3mutation or gene fusion within the tumor specimen is necessary to identify patients for whom treatment is indicated [see Dosage and Administration (2.1)] .
Ocular Disorders
Advise patients to contact their healthcare provider if they experience any visual changes [see Warnings and Precautions (5.1)]. In order to prevent or treat dry eyes, advise patients to use artificial tear substitutes, hydrating or lubricating eye gels or ointments frequently, at least every 2 hours during waking hours [see Dosage and Administration (2.3)].
Skin, Mucous or Nail Disorders
Advise patients to contact their healthcare provider if they experience progressive or intolerable skin, mucous or nail disorders [see Adverse Reactions (6.1)] .
Hyperphosphatemia and Soft Tissue Mineralization
Inform patients that BALVERSA may cause hyperphosphatemia and soft tissue mineralization. Advise patients to immediately inform their healthcare provider of painful skin lesions or any symptoms related to acute change in phosphate levels such as muscle cramps, numbness, or tingling around the mouth [see Warnings and Precautions (5.2)] .
Advise patients that their healthcare provider will assess their serum phosphate level between 14 and 21 days of initiating treatment and will adjust the dose if needed [see Warnings and Precautions (5.2)] . Advise patients to restrict phosphate intake to 600–800 mg daily. During this initial phosphate-assessment period, advise patients to avoid concomitant use with agents that can alter serum phosphate levels. Advise patients that, after the initial phosphate assessment period, monthly phosphate level monitoring for hyperphosphatemia should be performed during treatment with BALVERSA [see Drug Interactions (7.1)].
Drug Interactions
Advise patients to inform their healthcare providers of all concomitant medications, including prescription medicines, over-the-counter drugs, and herbal products [see Drug Interactions (7.1, 7.2)] .
Dosing Instructions
Instruct patients to swallow the tablets whole once daily with or without food. If vomiting occurs any time after taking BALVERSA, advise patients to take the next dose the next day [see Dosage and Administration (2.2)] .
Missed Dose
If a dose is missed, advise patients to take the missed dose as soon as possible. Resume the regular daily dose schedule for BALVERSA the next day. Extra tablets should not be taken to make up for the missed dose [see Dosage and Administration (2.3)] .
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to the fetus. Advise females to inform their healthcare providers of a known or suspected pregnancy [see Warnings and Precautions (5.3)and Use in Specific Populations (8.1)] .
Advise female patients of reproductive potential to use effective contraception during treatment and for one month after the last dose of BALVERSA. Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for one month after the last dose of BALVERSA [see Use in Specific Populations (8.3)].
Lactation
Advise females not to breastfeed during treatment with BALVERSA and for one month after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise females of reproductive potential that BALVERSA may impair fertility [see Use in Specific Populations (8.3)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
BALVERSA ®(bal-VER-sah)
(erdafitinib) tabletsThis Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 01/2024 What is BALVERSA?
BALVERSA is a prescription medicine used to treat adults with bladder cancer (urothelial cancer) that has spread or cannot be removed by surgery:- which has a certain type of abnormal FGFR gene, and
- who have tried at least one other medicine by mouth or injection (systemic therapy) that did not work or is no longer working.
BALVERSA is not recommended for the treatment of people who are eligible for and have not received prior PD-1 or PD-L1 inhibitor therapy.
It is not known if BALVERSA is safe and effective in children.Before taking BALVERSA tell your healthcare provider about all of your medical conditions, including if you: - have vision or eye problems.
- are pregnant or plan to become pregnant. BALVERSA can harm your unborn baby. You should not become pregnant during treatment with BALVERSA.
Females who can become pregnant:- Your healthcare provider may do a pregnancy test before you start treatment with BALVERSA.
- You should use effective birth control during treatment and for 1 month after the last dose of BALVERSA. Talk to your healthcare provider about birth control methods that may be right for you.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant.
- You should use effective birth control when sexually active during treatment with BALVERSA and for 1 month after the last dose.
- are breastfeeding or plan to breastfeed. Do not breastfeed during treatment and for 1 month after the last dose of BALVERSA.
How should I take BALVERSA? - Take BALVERSA exactly as your healthcare provider tells you.
- Take BALVERSA 1 time each day.
- Swallow BALVERSA tablets whole with or without food.
- Your healthcare provider may change your dose of BALVERSA, temporarily stop or completely stop treatment if you get certain side effects.
- If you miss a dose of BALVERSA, take the missed dose as soon as possible on the same day. Take your regular dose of BALVERSA the next day. Do not take more BALVERSA than prescribed to make up for the missed dose.
- If you vomit after taking BALVERSA, do not take another BALVERSA tablet. Take your regular dose of BALVERSA the next day.
What are the possible side effects of BALVERSA?
BALVERSA may cause serious side effects, including:- Eye problems.Eye problems are common with BALVERSA but can also be serious. Eye problems include dry or inflamed eyes, inflamed cornea (front part of the eye) and disorders of the retina, an internal part of the eye. Tell your healthcare provider right away if you develop blurred vision, loss of vision or other visual changes. You should use artificial tear substitutes, hydrating or lubricating eye gels or ointments at least every 2 hours during waking hours to help prevent dry eyes. During treatment with BALVERSA, your healthcare provider will send you to see an eye specialist.
-
High phosphate levels in the blood (hyperphosphatemia).Hyperphosphatemia is common with BALVERSA but can also be serious. High levels of phosphate in your blood may lead to build-up of minerals such as calcium in different tissues in your body. Your healthcare provider will check your blood phosphate level between 14 and 21 days after starting treatment with BALVERSA, and then monthly.
- Your healthcare provider may prescribe changes in your diet or phosphate lowering therapy, or change or stop treatment with BALVERSA if needed.
- Tell your healthcare provider right away if you develop painful skin lesions, any muscle cramps, or numbness or tingling around your mouth.
- nails separate from the bed or poor formation of the nail
- mouth sores
- diarrhea
- increased level of creatinine in the blood
- increased level of the enzyme alkaline phosphatase in the blood
- change in liver function
- decreased red blood cells (anemia)
- decreased salt (sodium) levels in the blood
- tiredness
- dry mouth
- dry skin
- decreased phosphate in the blood
- decreased appetite
- change in sense of taste
- constipation
- increased level of calcium in the blood
- dry eye
- redness, swelling, peeling or tenderness, mainly on the hands or feet (hand-foot syndrome)
- increased level of potassium in the blood
- hair loss
- fluid buildup behind the retina in your eye
Tell your healthcare provider right away if you develop any nail or skin problems including nails separating from the nail bed, nail pain, nail bleeding, breaking of the nails, color or texture changes in your nails, infected skin around the nail, an itchy skin rash, dry skin, or cracks in the skin.
BALVERSA may affect fertility in females who are able to become pregnant. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of BALVERSA. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store BALVERSA? - Store BALVERSA tablets at room temperature between 68°F to 77°F (20°C to 25°C).
General information about the safe and effective use of BALVERSA.
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use BALVERSA for a condition for which it was not prescribed. Do not give BALVERSA to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about BALVERSA that is written for healthcare professionals.What are the ingredients in BALVERSA?
Active ingredient:erdafitinib
Inactive ingredients:
Tablet Core: Croscarmellose sodium, Magnesium stearate (from vegetable source), Mannitol, Meglumine, and Microcrystalline Cellulose.
Film Coating (Opadry amb II): Glycerol monocaprylocaprate Type I, Polyvinyl alcohol-partially hydrolyzed, Sodium lauryl sulfate, Talc, Titanium dioxide, Iron oxide yellow, Iron oxide red (for the orange and brown tablets only), Ferrosoferric oxide/iron oxide black (for the brown tablets only).
Manufactured for:Janssen Products, LP, Horsham, PA 19044, USA
For patent information: www.janssenpatents.com
© 2019 Janssen Pharmaceutical Companies
For more information call Janssen Products, LP at 1-800-526-7736 (1-800-JANSSEN) or go to www.BALVERSA.com. - PRINCIPAL DISPLAY PANEL - 3 mg Tablet Bottle Carton
- PRINCIPAL DISPLAY PANEL - 4 mg Tablet Bottle Carton
- PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle Carton
-
INGREDIENTS AND APPEARANCE
BALVERSA
erdafitinib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59676-030 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERDAFITINIB (UNII: 890E37NHMV) (ERDAFITINIB - UNII:890E37NHMV) ERDAFITINIB 3 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) MEGLUMINE (UNII: 6HG8UB2MUY) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) GLYCERYL MONOCAPRYLOCAPRATE (UNII: G7515SW10N) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color yellow Score no score Shape OVAL (biconvex shaped) Size 8mm Flavor Imprint Code 3;EF Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59676-030-56 1 in 1 CARTON 04/12/2019 1 56 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:59676-030-84 1 in 1 CARTON 04/12/2019 2 84 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212018 04/12/2019 BALVERSA
erdafitinib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59676-040 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERDAFITINIB (UNII: 890E37NHMV) (ERDAFITINIB - UNII:890E37NHMV) ERDAFITINIB 4 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) MEGLUMINE (UNII: 6HG8UB2MUY) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) GLYCERYL MONOCAPRYLOCAPRATE (UNII: G7515SW10N) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color orange Score no score Shape OVAL (biconvex shaped) Size 8mm Flavor Imprint Code 4;EF Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59676-040-28 1 in 1 CARTON 04/12/2019 1 28 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:59676-040-56 1 in 1 CARTON 04/12/2019 2 56 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212018 04/12/2019 BALVERSA
erdafitinib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59676-050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERDAFITINIB (UNII: 890E37NHMV) (ERDAFITINIB - UNII:890E37NHMV) ERDAFITINIB 5 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) MEGLUMINE (UNII: 6HG8UB2MUY) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) GLYCERYL MONOCAPRYLOCAPRATE (UNII: G7515SW10N) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color brown Score no score Shape OVAL (biconvex shaped) Size 9mm Flavor Imprint Code 5;EF Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59676-050-28 1 in 1 CARTON 04/12/2019 1 28 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212018 04/12/2019 Labeler - Janssen Products LP (804684207) Establishment Name Address ID/FEI Business Operations Cilag AG 483237103 api manufacture(59676-030, 59676-040, 59676-050) Establishment Name Address ID/FEI Business Operations Johnson & Johnson Private Limited 677603030 analysis(59676-030, 59676-040, 59676-050) Establishment Name Address ID/FEI Business Operations Janssen Cilag SpA 542797928 manufacture(59676-030, 59676-040, 59676-050) , analysis(59676-030, 59676-040, 59676-050) , pack(59676-030, 59676-040, 59676-050)

 NDC 59676-030-56
NDC 59676-030-56
 NDC 59676-040-56
NDC 59676-040-56
 NDC 59676-050-28
NDC 59676-050-28