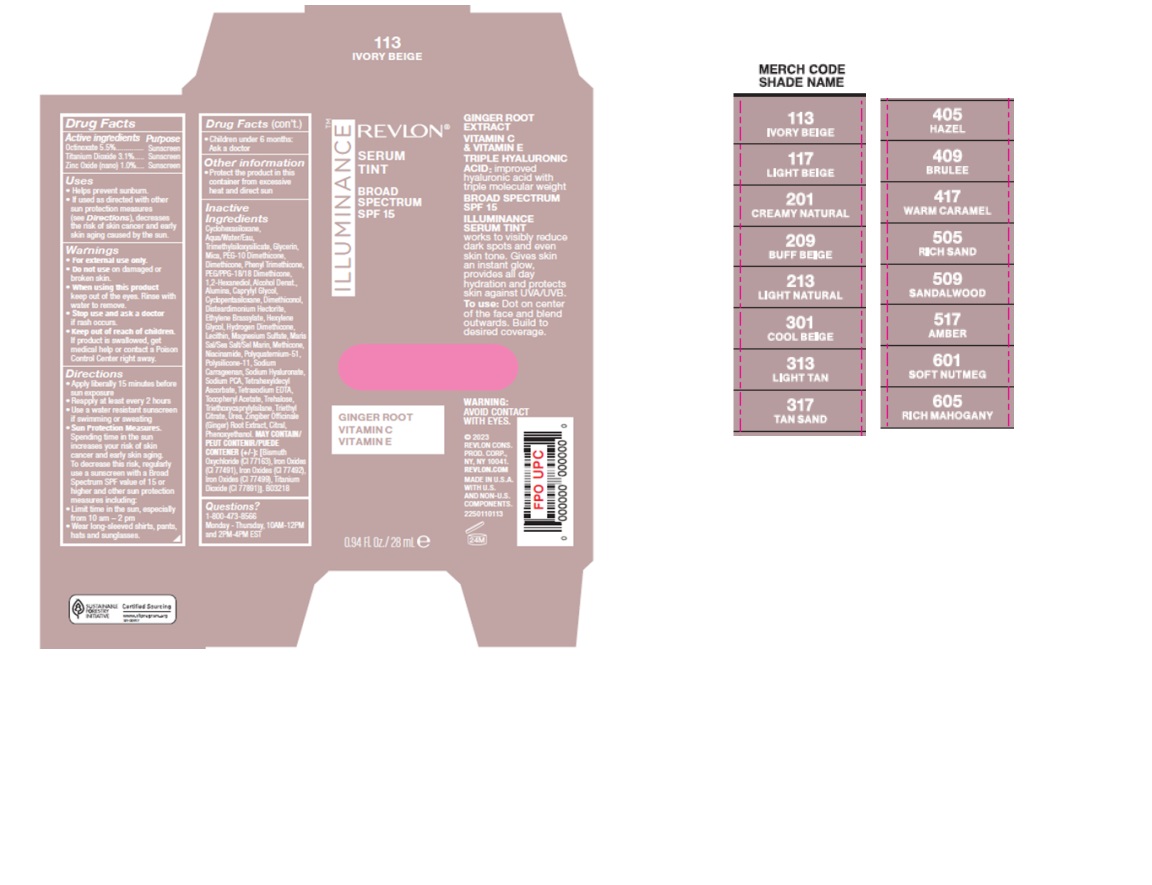
Label: RV ILLUMINANCE SERUM TINT BROAD SPECTRUM 15 CREAMY NATURAL- face foundation with spf cream
- NDC Code(s): 10967-696-19
- Packager: REVLON CONSUMER PR Consumer Products Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- Uses
- Warnings
-
Directions
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.–2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
Inactive Ingredients: Cyclohexasiloxane, Aqua/Water/Eau, Trimethylsiloxysilicate, Glycerin, Mica, PEG-10 Dimethicone, Dimethicone, Phenyl Trimethicone, PEG/PPG-18/18 Dimethicone, 1,2-Hexanediol, Alcohol Denat., Alumina, Caprylyl Glycol, Cyclopentasiloxane, Dimethiconol, Disteardimonium Hectorite, Ethylene Brassylate, Hexylene Glycol, Hydrogen Dimethicone, Lecithin, Magnesium Sulfate, Maris Sal/Sea Salt/Sel Marin, Methicone, Niacinamide, Polyquaternium-51, Polysilicone-11, Sodium Carrageenan, Sodium Hyaluronate, Sodium PCA, Tetrahexyldecyl Ascorbate, Tetrasodium EDTA, Tocopheryl Acetate, Trehalose, Triethoxycaprylylsilane, Triethyl Citrate, Urea, Zingiber Officinale (Ginger) Root Extract, Citral, Phenoxyethanol.
MAY CONTAIN/PEUT CONTENIR/PUEDE CONTENER (+/-): [Bismuth Oxychloride (CI 77163), Iron Oxides (CI 77491), Iron Oxides (CI 77492), Iron Oxides (CI 77499), Titanium Dioxide (CI 77891).]. B03218
- Questions?
- Keep out of reach of children
- Purpose
- Carton Packaging
- Tube Packaging Label
-
INGREDIENTS AND APPEARANCE
RV ILLUMINANCE SERUM TINT BROAD SPECTRUM 15 CREAMY NATURAL
face foundation with spf creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-696 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.868 g in 28 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.28 g in 28 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.54 g in 28 mL Inactive Ingredients Ingredient Name Strength TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) GLYCERIN (UNII: PDC6A3C0OX) MICA (UNII: V8A1AW0880) CYCLOMETHICONE 6 (UNII: XHK3U310BA) DIMETHICONE (UNII: 92RU3N3Y1O) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) EDETATE SODIUM (UNII: MP1J8420LU) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) UREA (UNII: 8W8T17847W) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) GINGER (UNII: C5529G5JPQ) CITRAL (UNII: T7EU0O9VPP) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONOL (100000 CST) (UNII: OSA9UP217S) HEXYLENE GLYCOL (UNII: KEH0A3F75J) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) ALCOHOL (UNII: 3K9958V90M) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) SEA SALT (UNII: 87GE52P74G) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) METHICONE (20 CST) (UNII: 6777U11MKT) NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CARRAGEENAN (UNII: 5C69YCD2YJ) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-696-19 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 03/26/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/26/2024 Labeler - REVLON CONSUMER PR Consumer Products Corp (788820165) Establishment Name Address ID/FEI Business Operations REVLON, INC. 809725570 manufacture(10967-696)