Label: MILBEGUARD FLAVORED- milbemycin oxime tablet
- NDC Code(s): 13744-510-10, 13744-511-10, 13744-512-10, 13744-513-10
- Packager: Ceva Sante Animale
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated April 26, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
MilbeGuard™
(milbemycin oxime)
Flavored Tablets
INFORMATION FOR DOSING DOGS
The once-a-month tablet that prevents heartworm disease, controls adult hookworm, and removes and controls adult roundworm and whipworm infections in dogs and puppies.
Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Keep this and all drugs out of the reach of children. -
DESCRIPTION
Description: MILBEGUARD (milbemycin oxime) Flavored Tablets are available in four tablet sizes in color-coded packages for oral administration to dogs and puppies. Each tablet is formulated to provide a minimum of 0.23 mg/lb (0.5 mg/kg) body weight of milbemycin oxime. Milbemycin oxime consists of the oxime derivatives of 5-didehydromilbemycins in the ratio of approximately 80% A4 (C32H45NO7, MW 555.71) and 20% A3 (C31H43NO7, MW 541.68).
Package Color Milbemycin oxime tablet Yellow 2.3 mg* Blue 5.75 mg Purple 11.5 mg Red 23.0 mg *for dogs only
-
INDICATIONS & USAGE
Indications: MILBEGUARD Flavored Tablets are indicated for use in the prevention of heartworm disease caused by Dirofilaria immitis, the control of adult Ancylostoma caninum (hookworm), and the removal and control of adult Toxocara canis and Toxascaris leonina (roundworms) and Trichuris vulpis (whipworm) infections in dogs and in puppies four weeks of age or greater and two pounds body weight or greater.
-
DOSAGE & ADMINISTRATION
Dosage: MILBEGUARD Flavored Tablets are given orally, once a month, at the recommended minimum dosage rate of 0.23 mg milbemycin oxime per pound of body weight (0.5 mg/kg).
Recommended Dosage Schedule for Dogs Body Weight MILBEGUARD Flavored Tablets 2-10 lbs. One tablet (2.3 mg) 11-25 lbs. One tablet (5.75 mg) 26-50 lbs. One tablet (11.5 mg) 51-100 lbs. One tablet (23.0 mg) Dogs over 100 Ibs. are provided the appropriate combination of tablets.
Administration: MILBEGUARD Flavored Tablets are dual-purpose and may be offered in food or administered as other tablet medications. Watch the dog closely following dosing to be sure the entire dose has been consumed. If it is not entirely consumed, redose once with the full
recommended dose as soon as possible.MILBEGUARD Flavored Tablets must be administered monthly, preferably on the same date each month. The first dose should be administered within one month of the dogs first exposure to mosquitoes and monthly thereafter until the end of the mosquito season. If a dose is missed and a 30-day interval between dosing is exceeded, administer MILBEGUARD Flavored Tablets immediately and resume the monthly dosing schedule.
If MILBEGUARD Flavored Tablets replaces diethylcarbamazine (DEC) for heartworm prevention, the first dose must be given within 30 days after the last dose of DEC.
-
PRECAUTIONS
Precautions: Do not use in puppies less than four weeks of age or less than two pounds of body weight. Prior to initiation of the MILBEGUARD Flavored Tablets treatment program, dogs should be tested for existing heartworm infections. Infected dogs should be treated to remove adult
heartworms and microfilariae prior to initiating treatment with MILBEGUARD Flavored Tablets. Mild, transient hypersensitivity reactions manifested as labored respiration, vomiting, salivation and lethargy, have been noted in some treated dogs carrying a high number of circulating microfilariae. These reactions are presumably caused by release of protein from dead or dying microfilariae. - ADVERSE REACTIONS
-
SUMMARY OF SAFETY AND EFFECTIVENESS
Efficacy: MILBEGUARD Flavored Tablets eliminate the tissue stage of heartworm larvae and the adult stage of hookworm (Ancylostoma caninum), roundworms (Toxocara canis, Toxascaris leonina) and whipworm (Trichuris vulpis) infestations when administered orally according to the recommended dosage schedule.The anthelmintic activity of milbemycin oxime is believed to be a result of interference with invertebrate neurotransmission.
Safety: Milbemycin oxime has been tested safely in over 75 different breeds of dogs, including collies, pregnant females, breeding males and females, and puppies over two weeks of age. In well-controlled clinical field studies, 786 dogs completed treatment with milbemycin oxime. Milbemycin oxime was used safely in animals receiving frequently used veterinary products such as vaccines, anthelmintics, antibiotics, steroids, flea collars, shampoos and dips.
Two studies in heartworm-infected dogs were conducted which demonstrated mild, transient hypersensitivity reactions in treated dogs with high microfilaremia counts (see Precautions for reactions observed). Safety studies in pregnant dogs demonstrated that high doses (1.5 mg/kg =3X) of milbemycin oxime given in an exaggerated dosing regimen (daily from mating through weaning), resulted in measurable concentrations of the drug in milk. Puppies nursing these females which received exaggerated dosing regimens demonstrated milbemycin-related effects. These effects were directly attributable to the exaggerated experimental dosing regimen. The product is normally intended for once-a-month administration only. Subsequent studies included using 3X daily from mating to one week before weaning and demonstrated no effects on the pregnant females or their litters. A second study where pregnant females were dosed once at 3X the monthly use rate either before, on the day of or shortly after whelping resulted in no effects on the puppies.
Some nursing puppies, at 2, 4, and 6 weeks of age, given greatly exaggerated oral milbemycin oxime doses (9.6 mg/kg = 19X) exhibited signs typified by tremors, vocalization and ataxia. These effects were all transient and puppies returned to normal within 24 to 48 hours. No effects were observed in puppies given the recommended dose of milbemycin oxime (0.5 mg/kg). This product has not been tested in dogs less than 1 kg weight.
A rising-dose safety study conducted in rough-coated collies, manifested a clinical reaction consisting of ataxia, pyrexia and periodic recumbency, in one of fourteen dogs treated with milbemycin oxime at 12.5 mg/kg (25X monthly use rate). Prior to receiving the 12.5 mg/kg dose (25X monthly use rate) on day 56 of the study, all animals had undergone an exaggerated dosing regimen consisting of 2.5 mg/kg milbemycin oxime (5X monthly use rate) on day 0, followed by 5.0 mg/kg (10X monthly use rate) on day 14 and 10.0 mg/kg (20X monthly use rate) on day 32. No
adverse reactions were observed in any of the collies treated with this regimen up through the 10.0 mg/kg (20X monthly use rate) dose. - HOW SUPPLIED
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
MilbeGuard™
(milbemycin oxime)
Flavored Tablets
INFORMATION FOR DOSING CATS
The once-a-month tablet that prevents heartworm disease and removes adult roundworms and hookworms in cats and kittens.
Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Keep this and all drugs out of the reach of children. -
DESCRIPTION
Description: MILBEGUARD (milbemycin oxime) Flavored Tablets for Cats are available in three tablet sizes in color-coded packages for oral administration to cats and kittens. Each tablet is formulated to provide a minimum of 0.9 mg/lb (2.0 mg/kg) body weight of milbemycin oxime. Milbemycin oxime consists of the oxime derivatives of 5-didehydromilbemycins in the ratio of approximately 80% A4 (C32H45NO7, MW 555.71) and 20% A3 (C31H43NO7, MW 541.68).
Package Color Milbemycin oxime tablet Blue 5.75 mg Purple 11.5 mg Red 23.0 mg -
INDICATIONS & USAGE
Indications: MILBEGUARD Flavored Tablets for Cats are indicated for use in the prevention of heartworm disease caused by Dirofilaria immitis, and the removal of adult Ancylostoma tubaeforme (hookworm) and Toxocara cati (roundworm) in cats and kittens six weeks of age or greater and 1.5 lbs. body weight or greater.
-
DOSAGE & ADMINISTRATION
Dosage: MILBEGUARD Flavored Tablets fort Cats are given orally, once a month, at the recommended minimum dosage rate of 0.9 mg milbemycin oxime per pound of body weight (2.0mg/kg).
Recommended Dosage Schedule for Cats Body Weight MILBEGUARD Flavored Tablets 1.5-6 lbs. One tablet (5.75 mg) 6.1-12 lbs. One tablet (11.5 mg) 12.1-25 lbs. One tablet (23.0 mg) Administration: MILBEGUARD Flavored Tablets for Cats may be offered in food or administered as other tablet medications. The tablets can be broken for ease of administration. Watch the cat closely following dosing to be sure the entire dose has been consumed. If it is not entirely consumed, redose once with the full recommended dose as soon as possible.
MILBEGUARD Flavored Tablets for Cats must be administered monthly, preferably on the same date each month. The first dose should be administered within one month of the cats first exposure to mosquitoes and monthly thereafter until the end of the mosquito season. If a dose is missed and a 30-day interval between dosing is exceeded, administer MILBEGUARD Flavored Tablets for Cats immediately and resume the monthly dosing schedule. It is recommended that cats be tested for existing heartworm infection prior to starting treatment with MILBEGUARD Flavored Tablets for Cats (See Precautions).
- PRECAUTIONS
-
SUMMARY OF SAFETY AND EFFECTIVENESS
Efficacy: MILBEGUARD Flavored Tablets for Cats eliminate the tissue stage of heartworm larvae and hookworm (Ancylostoma tubaeforme) and roundworm (Toxocara cati) infections when administered orally according to the recommended dosage schedule. The anthelmintic activity of milbemycin oxime is believed to be a result of interference with invertebrate neurotransmission.
Safety: Milbemycin oxime has been tested safely in over 8 different breeds of cats. In well-controlled clinical field studies 141 cats completed treatment with milbemycin oxime. Milbemycin oxime was used safely in animals receiving frequently used veterinary products such as vaccines, anthelmintics, anesthetics, antibiotics, steroids, flea collars, shampoos and dips.
Safety studies were conducted in young cats and kittens and doses of 1X, 3X and 5X the minimum recommended dose of 2.0 mg/kg demonstrated no drug- related effects. Tolerability studies at exaggerated doses of 10X also demonstrated no drug-related adverse effects in kittens and young adult cats.
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 2.3 mg Tablet Blister Pack
-
PRINCIPAL DISPLAY PANEL - 2.3 mg Carton
MilbeGuard™
(milbemycin oxime)
Flavored Tablets
BROAD SPECTRUM PARASITICIDE
Contains once-a-month Flavored Tablets to prevent heartworm disease, control adult hookworm infeaction, and remove and control adult roundworms and whipworms in dogs and puppies.
For dogs 2-10 lbs.
Net contents: 6 tablets 2.3 mg each.
ANADA #200-629, Approved by FDA
Keep this and all drugs out of the reach of children.
Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Keep this and all drugs out of the reach of children.Give monthly
6 pack

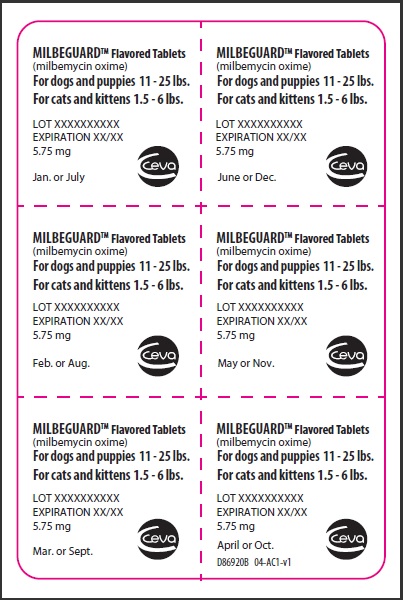
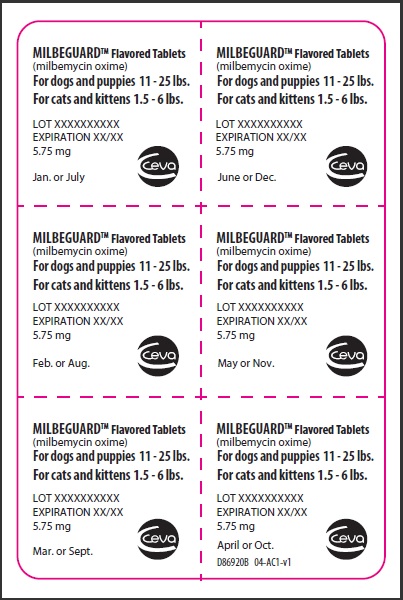
- PRINCIPAL DISPLAY PANEL - 5.75 mg Tablet Blister Pack
-
PRINCIPAL DISPLAY PANEL - 5.75 mg Carton
MilbeGuard™
(milbemycin oxime)
Flavored Tablets
BROAD SPECTRUM PARASITICIDE
Contains once-a-month Flavored Tablets to prevent heartworm disease, control adult hookworm infeaction, and remove and control adult roundworms and whipworms in dogs and puppies, and to prevent heartworm disease and remove adult hookworms and roundworms in cats and kittens.
For dogs 11-25 lbs.
For cats 1.5-6lbs.
Net contents: 6 tablets 5.75 mg each.
ANADA #200-629, Approved by FDA
Keep this and all drugs out of the reach of children.
Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Keep this and all drugs out of the reach of children.Give monthly
6 pack

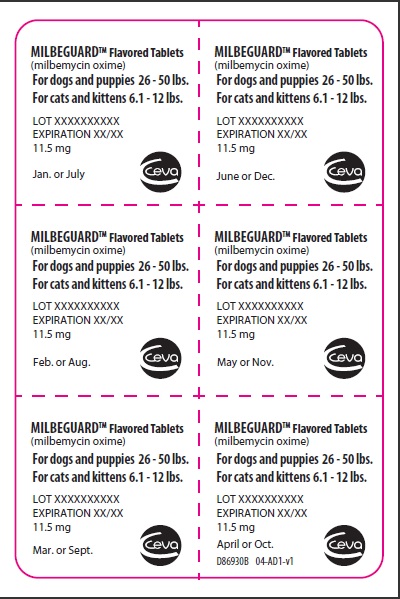
- PRINCIPAL DISPLAY PANEL - 11.5 mg Tablet Blister Pack
-
PRINCIPAL DISPLAY PANEL - 11.5 mg Carton
MilbeGuard™
(milbemycin oxime)
Flavored Tablets
BROAD SPECTRUM PARASITICIDE
Contains once-a-month Flavored Tablets to prevent heartworm disease, control adult hookworm infeaction, and remove and control adult roundworms and whipworms in dogs and puppies, and to prevent heartworm disease and remove adult hookworms and roundworms in cats and kittens.
For dogs 26-50 lbs.
For cats 6.1-12 lbs.
Net contents: 6 tablets 11.5 mg each.
ANADA #200-629, Approved by FDA
Keep this and all drugs out of the reach of children.
Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Keep this and all drugs out of the reach of children.Give monthly
6 pack

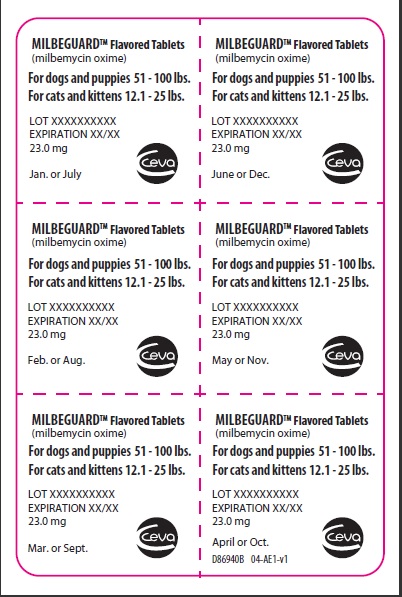
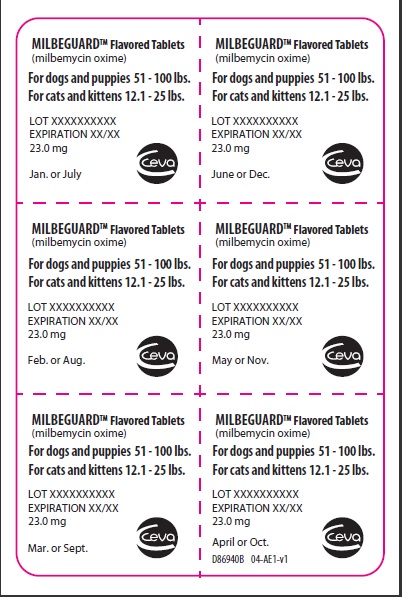
- PRINCIPAL DISPLAY PANEL - 23 mg Blister Pack
-
PRINCIPAL DISPLAY PANEL - 23 mg Carton
MilbeGuard™
(milbemycin oxime)
Flavored Tablets
BROAD SPECTRUM PARASITICIDE
Contains once-a-month Flavored Tablets to prevent heartworm disease, control adult hookworm infeaction, and remove and control adult roundworms and whipworms in dogs and puppies, and to prevent heartworm disease and remove adult hookworms and roundworms in cats and kittens.
For dogs 51-100 lbs.
For cats 12.1-25 lbs.
Net contents: 6 tablets 23.0 mg each.
ANADA #200-629, Approved by FDA
Keep this and all drugs out of the reach of children.
Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Keep this and all drugs out of the reach of children.Give monthly
6 pack

-
INGREDIENTS AND APPEARANCE
MILBEGUARD FLAVORED
milbemycin oxime tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13744-510 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MILBEMYCIN OXIME (UNII: 0502PUN0GT) (MILBEMYCIN OXIME - UNII:0502PUN0GT) MILBEMYCIN OXIME 2.3 mg Product Characteristics Color brown (beige mottled) Score no score Shape ROUND Size 6mm Flavor MEAT Imprint Code PAH;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13744-510-10 1 in 1 CARTON 1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200629 12/21/2018 MILBEGUARD FLAVORED
milbemycin oxime tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13744-511 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MILBEMYCIN OXIME (UNII: 0502PUN0GT) (MILBEMYCIN OXIME - UNII:0502PUN0GT) MILBEMYCIN OXIME 5.75 mg Product Characteristics Color brown (beige mottled) Score no score Shape ROUND Size 8mm Flavor MEAT Imprint Code PAH;6 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13744-511-10 1 in 1 CARTON 1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200629 12/21/2018 MILBEGUARD FLAVORED
milbemycin oxime tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13744-512 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MILBEMYCIN OXIME (UNII: 0502PUN0GT) (MILBEMYCIN OXIME - UNII:0502PUN0GT) MILBEMYCIN OXIME 11.5 mg Product Characteristics Color brown (beige mottled) Score no score Shape ROUND Size 10mm Flavor MEAT Imprint Code PAH;12 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13744-512-10 1 in 1 CARTON 1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200629 12/21/2018 MILBEGUARD FLAVORED
milbemycin oxime tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13744-513 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MILBEMYCIN OXIME (UNII: 0502PUN0GT) (MILBEMYCIN OXIME - UNII:0502PUN0GT) MILBEMYCIN OXIME 23 mg Product Characteristics Color brown (beige mottled) Score no score Shape ROUND Size 16mm Flavor MEAT Imprint Code PAH;23 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13744-513-10 1 in 1 CARTON 1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200629 12/21/2018 Labeler - Ceva Sante Animale (261126049)