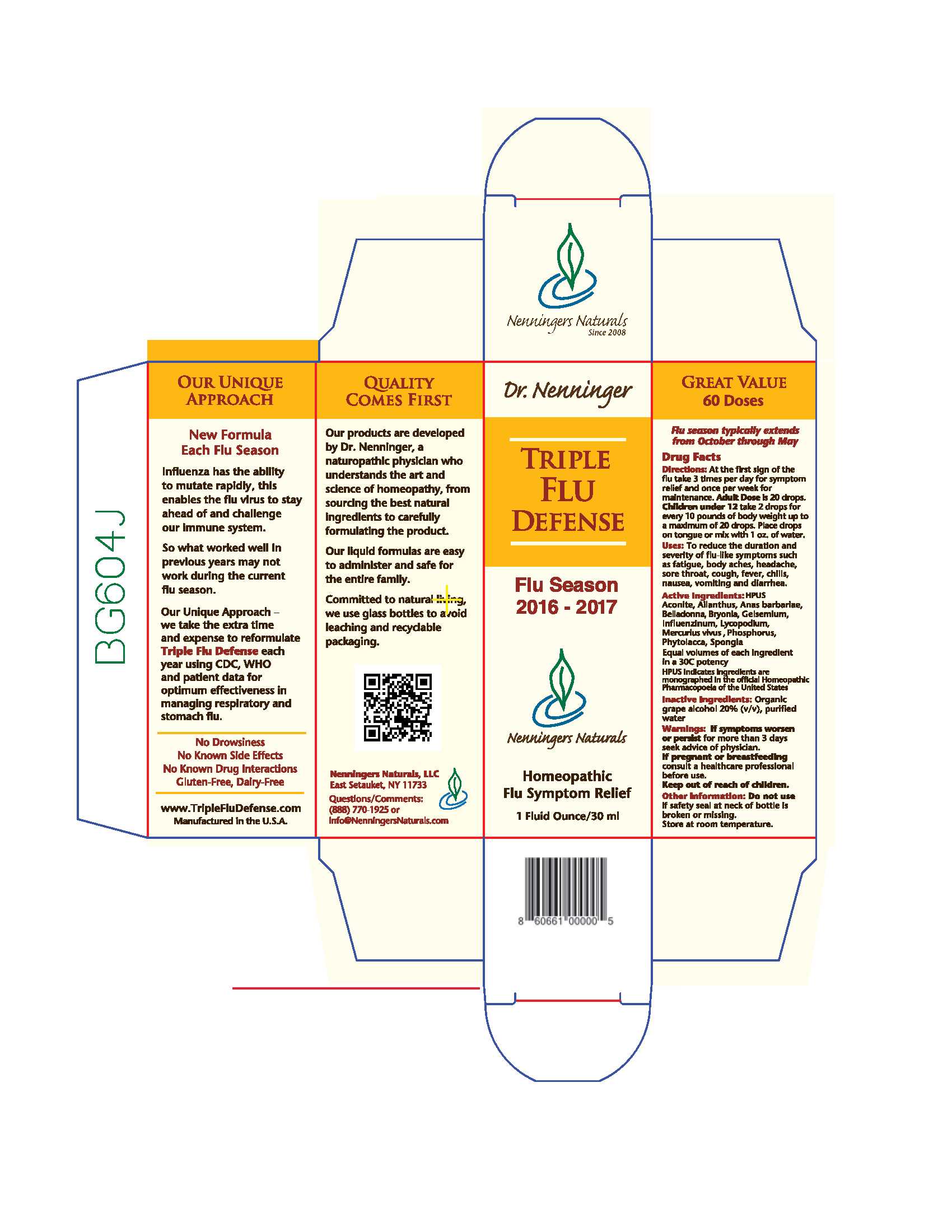
Label: TRIPLE FLU DEFENSE- aconite, ailanthus, anas barbariae, belladonna, bryonia, gelsemium, influenzinum, lycopodium, mercurius vivus, phosphorus, phytolacca, spongia liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 42731-022-01 - Packager: Nenningers Naturals, LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 7, 2017
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- INDICATIONS & USAGE
-
ACTIVE INGREDIENT
Active Ingredients: HPUS
Aconite, Ailanthus, Anas barbariae, Belladonna, Bryonia, Gelsemium, Influenzinum, Lycopodium, Mercurius vivus, Phosphorus, Phytolacca, Spongia
Equal volumes of each ingredient in a 30C potency.
HPUS indicates ingredients are monographed in th official Homeopathic Pharmacopeia of the United States
- INACTIVE INGREDIENT
- WARNINGS
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRIPLE FLU DEFENSE
aconite, ailanthus, anas barbariae, belladonna, bryonia, gelsemium, influenzinum, lycopodium, mercurius vivus, phosphorus, phytolacca, spongia liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42731-022 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS ROOT (UNII: KPD2N7348X) (ACONITUM NAPELLUS ROOT - UNII:KPD2N7348X) ACONITUM NAPELLUS ROOT 30 [hp_C] in 30 mL AILANTHUS ALTISSIMA FLOWERING TWIG (UNII: 8P29O5P7XU) (AILANTHUS ALTISSIMA FLOWERING TWIG - UNII:8P29O5P7XU) AILANTHUS ALTISSIMA FLOWERING TWIG 30 [hp_C] in 30 mL CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE (UNII: RN2HC612GY) (CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE - UNII:RN2HC612GY) CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE 30 [hp_C] in 30 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] in 30 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 30 [hp_C] in 30 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 30 [hp_C] in 30 mL INFLUENZA A VIRUS A/CALIFORNIA/7/2009 (H1N1)-LIKE HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) (UNII: K9P8PVA2UG) (INFLUENZA A VIRUS A/CALIFORNIA/7/2009 (H1N1)-LIKE HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) - UNII:K9P8PVA2UG) INFLUENZA A VIRUS A/CALIFORNIA/7/2009 (H1N1)-LIKE HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) 30 [hp_C] in 30 mL INFLUENZA B VIRUS B/PHUKET/3073/2013 HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) (UNII: 8UK4958MZX) (INFLUENZA B VIRUS B/PHUKET/3073/2013 HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) - UNII:8UK4958MZX) INFLUENZA B VIRUS B/PHUKET/3073/2013 HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) 30 [hp_C] in 30 mL INFLUENZA A VIRUS A/SWITZERLAND/9715293/2013 NIB-88 (H3N2) HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) (UNII: H1SQ9X1EF0) (INFLUENZA A VIRUS A/SWITZERLAND/9715293/2013 NIB-88 (H3N2) HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) - UNII:H1SQ9X1EF0) INFLUENZA A VIRUS A/SWITZERLAND/9715293/2013 NIB-88 (H3N2) HEMAGGLUTININ ANTIGEN (PROPIOLACTONE INACTIVATED) 30 [hp_C] in 30 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_C] in 30 mL MERCURY (UNII: FXS1BY2PGL) (MERCURY - UNII:FXS1BY2PGL) MERCURY 30 [hp_C] in 30 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_C] in 30 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 30 [hp_C] in 30 mL SPONGIA OFFICINALIS SKELETON, ROASTED (UNII: 1PIP394IID) (SPONGIA OFFICINALIS SKELETON, ROASTED - UNII:1PIP394IID) SPONGIA OFFICINALIS SKELETON, ROASTED 30 [hp_C] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42731-022-01 1 in 1 CARTON 09/01/2016 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/01/2016 Labeler - Nenningers Naturals, LLC (967048666)